The mechanisms underlying functional mitral regurgitation (MR) and the relation between mechanism and severity of MR have not been evaluated in a large, multicenter, randomized controlled trial. Transesophageal echocardiography (TEE) was performed in 215 patients at 17 centers in the Surgical Treatment for Ischemic Heart Failure (STICH) trial. Both 2-dimensional (n = 215) and 3-dimensional (n = 81) TEEs were used to assess multiple quantitative measurements of the mechanism and severity of MR. By 2-dimensional TEE, leaflet tenting area, anterior and posterior leaflet angles, mitral annulus diameter, left ventricular (LV) end-systolic volume index, LV ejection fraction (LVEF), and sphericity index (p <0.05 for all) were significantly different across MR grades. By 3-dimensional TEE, mitral annulus area, leaflet tenting area, LV end-systolic volume index, LVEF, and sphericity index (p <0.05 for all) were significantly different across MR grades. A multivariate analysis showed a trend for annulus area (p = 0.069) and LV end-systolic volume index (p = 0.071) to predict effective regurgitant orifice area and for annulus area (p = 0.018) and LV end-systolic volume index (p = 0.073) to predict vena contracta area. In the STICH trial, multiple quantitative parameters of the mechanism of functional MR are related to MR severity. The mechanism of functional MR in ischemic cardiomyopathy is heterogenous, but no single variable stands out as a strong predictor of quantitative severity of MR.
Functional mitral regurgitation (MR) is a common complication of ischemic heart disease, which is associated with increased mortality. Early studies of the mechanism of functional MR focused on mitral annular dilation and loss of systolic annular contraction due to left ventricular (LV) dilation, diminished mitral leaflet closing force, and abnormal LV shape. More recently, it has been shown that functional MR is caused by leaflet tethering as the papillary muscles are displaced apically and laterally by LV dilation. Papillary muscle displacement may be due to global or regional wall motion abnormalities and may be symmetric or asymmetric. Understanding the mechanisms of functional MR in any given patient has important implications regarding the correct approach to repair it. To date, neither large prospective studies have examined the mechanism(s) underlying functional MR in ischemic cardiomyopathy nor has the relation between mechanism and severity of functional MR been determined. This is a report of a prospectively defined ancillary study to the Surgical Treatment for Ischemic Heart Failure (STICH) trial, in which 2-dimensional (2D) and 3-dimensional (3D) transesophageal echocardiographies (TEE) were used to define the mechanism(s) of functional MR in a large clinical trial of patients with ischemic cardiomyopathy randomized to medical therapy, coronary bypass grafting, or coronary bypass grafting plus surgical ventricular restoration.
Methods
All participating STICH study sites were invited to participate in the STICH MR TEE substudy; 17 accepted. All 17 sites were provided with a detailed study protocol for obtaining TEE images to define the mechanism and severity of functional MR. An Institutional Review Board approval was obtained from each site, and written informed consent was obtained from all patients. At the time the STICH trial was initiated, real-time 3D imaging was not available. Sites equipped with a Philips ultrasound machine (Philips Ultrasound, Andover, Massachusetts) were capable of 3D TEE acquisitions using a rotational reconstruction algorithm. Philips graciously provided the 3D software to those sites. For patients in STICH trial randomized to medical therapy, TEE was done within 1 week of randomization. For patients randomized to surgery, a baseline preoperative TEE was done within 1 week before surgery. Intraoperative TEE was excluded by the study protocol because general anesthesia dramatically reduces the severity of MR by TEE.
TEE was performed during intravenous conscious sedation and local oropharyngeal anesthesia. Heart rate, blood pressure, oxygen saturation, and electrocardiogram were monitored throughout the procedure. Transgastric short-axis views of the LV were obtained at the midpapillary muscle and mitral leaflet levels. A transgastric long-axis view was also oriented to show both papillary muscles with their chordal attachments to the mitral leaflets. From the midesophagus, the probe was retroflexed to obtain a 4-chamber view with care to maximize LV cavity length and width while keeping the LV long axis in the center of the imaging sector. From this position, the imaging plane was manually rotated to obtain a commissural view, a 2-chamber view, and a long-axis view. For sites with 3D capability, at least two 3D rotational scans were obtained in each patient. Translational artifacts secondary to patient respiration were minimized using a proprietary software to automatically capture images within a defined respiratory threshold. After obtaining a well-aligned 4-chamber view, the probe was rotated automatically in 6° increments by the 3D TEE software across a total of 180°. These images were downloaded onto a 5.25″ magneto-optic disk and sent to the core laboratory, where analysis was performed in a blinded fashion using a custom software (Omni 4D; Massachusetts General Hospital, Boston, Massachusetts). This offered several advantages with regard to quality control. First, this software has been previously validated in vitro and in vivo. Second, it allowed the different TEE operators at different STICH sites to get consistent high-quality data from a single midesophageal probe position during quiet respiration or breath hold. Third, it allowed more accurate measurements without confounding by foreshortened or off-axis views or flattening of 3D measurements onto a 2D display screen.
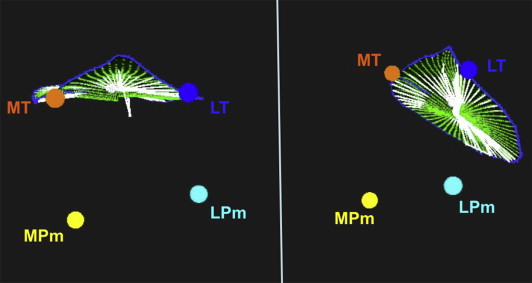
The mechanism(s) of functional MR was assessed by quantitative measurements including leaflet tethering distance, tethering angle, tenting area, papillary muscle displacement, and annulus area at end-diastole and end-systole. Three-dimensional TEE data sets were analyzed using the method by Otsuji et al. Manual tracing was used to identify the hinge points of the mitral leaflet insertion to identify the mitral annulus in each rotational imaging plane. The aortic annulus was identified by the hinge points of aortic leaflet insertion, and the intersection of the aortic and mitral annuli allowed identification of the medial and lateral fibrous trigones. The tips of the papillary muscles were also identified. In cases with complex papillary muscle anatomy, the largest papillary muscle head, which was most centrally located, was selected. All these points were assigned different colors by the Omni 4D software so that they could be tracked in 3D ( Figure 1 ). The computer allowed rotation of the images to facilitate analysis. The software then automatically calculated the mitral annulus areas at end-diastole and end-systole, the percent systolic contraction of the mitral annulus, the papillary muscle separation distance, the mitral tenting area and height, the papillary muscle separation angle (angle from the posteromedial papillary muscle to the medial trigone to the anterolateral papillary muscle), and the distances between the medial trigone and the posteromedial and anterolateral papillary muscles.
In addition to the mechanistic variables measured from TEE images, core laboratory evaluations of LV end-diastolic volume index and end-systolic volume index, LV ejection fraction (LVEF), and sphericity index were available from the STICH trial database. In the main STICH trial, all patients underwent baseline echocardiography, whereas magnetic resonance imaging and radionuclide imaging were optional. Using methods described previously, optimal LV volumes and LVEF were determined using an approach that incorporated all methods with the best correlation to overall mortality.
Quantitative measurements of MR severity, effective regurgitant orifice area (EROA), and vena contracta width (VCW) were used. MR was considered to be absent if no color flow signal was detectable superior to the mitral coaptation line during systole by color flow mapping. Trace MR was considered to be present when a few color pixels were present but no defined jet morphology was observed. For purposes of data analysis, none and trace MRs were grouped together. If a defined systolic color flow jet was present, the severity of MR was classified using a hierarchical approach. Accordingly, EROA by the proximal isovelocity surface area method was used to classify MR severity, unless it was of poor technical quality or could not be measured. If no MR was present, EROA was assigned a value of zero. As per the guidelines of the American Society of Echocardiography and the European Association of Echocardiography, mild MR was considered to be an EROA of ≤0.2 cm 2 , moderate MR 0.2 to 0.39 cm 2 , and severe MR ≥ 0.4 cm 2 . If EROA was not available or measureable, VCW was next used to classify MR severity with <0.3 cm being mild MR and ≥0.7 cm being severe MR. If neither EROA nor VCW were available, regurgitant volume by quantitative Doppler was used. If no quantitative measurements were available, the size of the color flow jet was used with ≤4.0 cm 2 denoting mild MR, and ≥8.0 cm 2 severe MR. Jet eccentricity, E-wave velocity, and pulmonary vein pattern were used to adjust the MR severity up or down by 1 grade using the integrative method described by the American Society of Echocardiography and the European Association of Echocardiography guidelines. All patients graded as moderate or severe MR had quantitative assessment of EROA.
Quantitative measurements of annulus size and leaflet tethering were compared in 3 groups of patients—those without MR, with mild MR (EROA <0.2 cm 2 ), and with at least moderate MR (EROA ≥0.2 cm 2 ). Specific measurements of MR mechanism were compared across MR grades. Analysis of variance was used to compare continuous variables of MR mechanism among the 3 groups of MR severity. Multivariate regression analysis was performed to evaluate which measurements of the mechanism of MR are independent predictors of MR severity as defined by (1) EROA and (2) VCW. For purposes of the multivariate analysis, EROA and VCW were assigned a value of 0 if MR was graded as none or trace.
Results
TEE studies were obtained in 214 subjects; of whom, 81 had both 2D and 3D TEEs performed and 134 had only 2D TEE. Of these, 210 studies (97.7%) were of sufficient quality to assess MR severity. There were 57 patients with no MR, 120 with mild, 29 with moderate, and 4 with severe MR. Of the subset of patients with 3D TEE, 26 had no MR, 44 had mild MR, and 11 had moderate or severe MR. Given the small sample of severe MR and the fact that even moderate MR portends a poor prognosis in heart failure, the moderate and severe MR grades were combined for statistical analysis.
Table 1 lists the demographic and clinical characteristics of the patients in the MR substudy compared with the STICH main trial population not included in the substudy. In general, patients in the MR substudy were similar to those in the parent trial, being predominantly white men with a median age of 60 years. There was a greater predominance of whites in the substudy compared with the main trial (91.1% vs 76.6%, p <0.0001), more patients without angina (37.4% vs 30.5%, p = 0.006), and more patients with New York Heart Association class I heart failure symptoms (20.1% vs 9.1%, p <0.0001). Patients in the MR substudy tended to have higher diastolic blood pressure (median of 80 vs 75 mm Hg, p = 0.016) and higher LV end-systolic volume index (median of 82.5 vs 77.9 ml/m 2 , p = 0.018). MR severity (as graded by the sites) tended to be slightly worse in patients in the MR substudy (moderate or severe MR in 27.8% vs 16.9%, p <0.001). Finally, patients in the MR substudy had slightly greater usage of the following medications: β blockers, angiotensin-converting enzyme inhibitors, diuretics, aspirin, aspirin or warfarin, and statins (p <0.05 for all).
| Variable | STICH H1/H2 Population | p Value | |
|---|---|---|---|
| MR Substudy (n = 214) | Not in MR Substudy (n = 1,922) | ||
| Age (yrs) | 60.4 (52.7, 68.0) | 60.5 (54.0, 68.1) | 0.317 |
| Men | 188/214 (87.9) | 1,662/1,922 (86.5) | 0.574 |
| Women | 26/214 (12.1) | 260/1,922 (13.5) | |
| White | 195/214 (91.1) | 1,473/1,922 (76.6) | <0.001 |
| Black | 5/214 (2.3) | 45/1,922 (2.3) | |
| Asian | 5/214 (2.3) | 235/1,922 (12.2) | |
| Other | 9/214 (4.2) | 164/1,922 (8.5) | |
| Multiracial | 5/1,922 (0.3) | ||
| Body mass index (kg/m 2 ) | 27.0 (24.5, 29.4) | 26.9 (24.2, 30.1) | 0.856 |
| Previous myocardial infarction | 183/214 (85.5) | 1,559/1,922 (81.1) | 0.115 |
| History of hyperlipidemia | 147/214 (68.7) | 1,245/1,918 (64.9) | 0.271 |
| History of hypertension | 129/214 (60.3) | 1,147/1,922 (59.7) | 0.865 |
| Diabetes mellitus | 70/214 (32.7) | 729/1,922 (37.9) | 0.134 |
| Current smoker | 39/214 (18.2) | 406/1,921 (21.1) | 0.320 |
| Previous percutaneous coronary intervention | 38/214 (17.8) | 294/1,922 (15.3) | 0.346 |
| Chronic renal insufficiency | 8/214 (3.7) | 164/1,920 (8.5) | 0.014 |
| Stroke | 18/214 (8.4) | 123/1,922 (6.4) | 0.261 |
| Previous coronary bypass | 2/214 (0.9) | 58/1,922 (3.0) | 0.080 |
| Current Canadian Cardiovascular Society angina class | 1.0 (0.0, 3.0) | 2.0 (0.0, 3.0) | 0.047 |
| None | 80/214 (37.4) | 586/1,922 (30.5) | 0.006 |
| I | 29/214 (13.6) | 216/1,922 (11.2) | |
| II | 51/214 (23.8) | 629/1,922 (32.7) | |
| III | 51/214 (23.8) | 400/1,922 (20.8) | |
| IV | 3/214 (1.4) | 91/1,922 (4.7) | |
| Current NYHA heart failure class | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | <0.001 |
| I | 43/214 (20.1) | 174/1,922 (9.1) | <0.001 |
| II | 111/214 (51.9) | 903/1,922 (47.0) | |
| III | 54/214 (25.2) | 765/1,922 (39.8) | |
| IV | 6/214 (2.8) | 80/1,922 (4.2) | |
| Systolic blood pressure (mm Hg) | 120.0 (110.0, 130.0) | 120.0 (110.0, 130.0) | 0.123 |
| Diastolic blood pressure (mm Hg) | 80.0 (70.0, 80.0) | 75.0 (69.0, 80.0) | 0.016 |
| Heart rate (beats/min) | 72.0 (66.0, 80.0) | 72.0 (65.0, 80.0) | 0.433 |
| Creatinine (mg/dl) | 1.1 (0.9, 1.2) | 1.1 (0.9, 1.3) | 0.067 |
| Baseline LVEF (%) CMR/RN/echo/site | 26.1 (20.2, 32.0) | 27.4 (22.0, 33.7) | 0.056 |
| End-systolic volume index (ml/m 2 ) | 82.5 (65.0, 103.2) | 77.9 (59.6, 98.6) | 0.018 |
| Anterior akinesia or dyskinesia (%) | 47.0 (29.0, 57.0) | 43.0 (35.0, 60.0) | 0.382 |
| MR severity grade ∗ | |||
| None or trace | 66/212 (31.1) | 702/1,912 (36.7) | <0.001 |
| Mild | 87/212 (41.0) | 886/1,912 (46.3) | |
| Moderate or severe | 59/212 (27.8) | 324/1,912 (16.9) | |
| Baseline medications | |||
| β blocker | 195/214 (91.1) | 1,631/1,922 (84.9) | 0.014 |
| ACE inhibitor or ARB | 193/214 (90.2) | 1,698/1,922 (88.3) | 0.423 |
| ACE inhibitor | 187/214 (87.4) | 1,550/1,922 (80.6) | 0.016 |
| Digoxin | 33/214 (15.4) | 351/1,922 (18.3) | 0.304 |
| Diuretic (potassium sparing) | 137/214 (64.0) | 752/1,922 (39.1) | <0.001 |
| Diuretic (loop/thiazide or potassium sparing) | 169/214 (79.0) | 1,360/1,922 (70.8) | 0.012 |
| Aspirin (daily) | 190/214 (88.8) | 1,521/1,922 (79.1) | <0.001 |
| Aspirin or warfarin | 197/214 (92.1) | 1,625/1,922 (84.5) | 0.003 |
| Statin | 195/214 (91.1) | 1,491/1,922 (77.6) | <0.001 |
∗ MR grade by transthoracic echocardiography as determined by study site investigators.
Table 2 lists the MR mechanistic variables by MR severity grades in the patients with 2D TEE studies. Multiple measurements were statistically significantly different across MR severity grades, including leaflet angle, posterior leaflet angle, mitral annulus diameter in the long-axis view (anteroposterior diameter), LV end-systolic volume index, LVEF, and sphericity index (p <0.05 for all). The most strongly predictive measurement of MR grade was LV end-systolic volume index (77 ml/m 2 for none or trace MR, 81 ml/m 2 for mild MR, and 99.9 ml/m 2 for moderate or severe MR, p <0.001).
| Variable | None or Trace (n = 57) | Mild (n = 120) | Moderate or Severe (n = 33) | p Value |
|---|---|---|---|---|
| Leaflet tenting area (cm 2 ) | 1.5 (1.2, 1.8) | 1.6 (1.4, 2.0) | 2.1 (1.4, 2.2) | 0.080 |
| Leaflet tenting height (cm) | 0.7 (0.6, 0.8) | 0.8 (0.7, 0.9) | 0.8 (0.7, 1.0) | 0.611 |
| Anterior leaflet angle (degrees) | 40.0 (29.0, 44.0) | 38.0 (32.0, 45.0) | 38.0 (29.0, 46.0) | 0.005 |
| Midanterior leaflet angle (degrees) | 148.0 (142.0, 158.0) | 149.0 (140.0, 158.0) | 147.0 (137.0, 158.0) | 0.122 |
| Posterior leaflet angle (degrees) | 55.0 (46.0, 65.0) | 54.0 (44.0, 64.0) | 54.0 (45.0, 69.0) | 0.028 |
| Annulus diameter 4-chamber view (cm) | 3.4 (3.3, 3.5) | 3.4 (3.1, 3.6) | 3.5 (3.2, 3.8) | 0.654 |
| Annulus diameter long-axis view (cm) | 3.2 (2.9, 3.4) | 3.3 (3.0, 3.5) | 3.5 (3.4, 3.7) | 0.004 |
| LV end-systolic volume index (ml/m 2 ) | 77.0 (57.3, 99.6) | 81.0 (66.3, 100.2) | 99.9 (82.6, 128.9) | <0.001 |
| Baseline LVEF (%) | 27.0 (21.8, 33.1) | 27.8 (20.0, 32.0) | 22.7 (18.8, 26.0) | 0.017 |
| Sphericity index | 1.4 (1.4, 1.6) | 1.4 (1.3, 1.6) | 1.3 (1.2, 1.4) | 0.008 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


