Mechanical Circulatory Support
Daniel B. Sims
Ulrich P. Jorde
Medical and electrical therapies have greatly improved the prognosis of patients with chronic heart failure (CHF). However, for patients with end-stage, refractory CHF (American College of Cardiology/American Heart Association Stage D CHF), medical and electrical therapies are insufficient. It is estimated that 0.2% of people aged >45 years in the United States have Stage D CHF,1 and 2 meaning that approximately 200,000 people could benefit from cardiac transplantation as the treatment for their disease. Unfortunately, approximately only 2,200 cardiac transplantations are performed each year in the United States because of a huge donor shortage. Owing to the epidemiologic limitations of cardiac transplantation, mechanical circulatory support has evolved to meet the needs of patients with Stage D CHF.
Mechanical circulatory support involves the use of a left ventricular assist device (LVAD) to improve hemodynamics. There are three main situations when LVADs are implanted: bridge to recovery, bridge to transplantation (BTT), and destination therapy (DT).
Bridge to recovery encompasses a clinical setting where there is an expectation of recovery of cardiac function. Examples include acute myocarditis, acute myocardial infarction, and postcardiotomy shock after open heart surgery.
BTT involves implanting an LVAD in patients who are listed for cardiac transplantation. Owing to the extended wait times for a donor organ, BTT patients need to have hemodynamic support with an LVAD prior to transplantation.
DT refers to the long-term placement of an LVAD in patients with Stage D CHF who are ineligible for cardiac transplantation. There was initial enthusiasm for the use of LVADs in patients with severe CHF to completely unload the LV and allow the initiation of high-dose CHF medications as well as the β2-adrenergic receptor agonist clenbuterol. In a small singlecenter study, this regimen led to the reversal of CHF and allowed LVAD explantation in 11 of 15 patients.3 Unfortunately, such a degree of myocardial improvement was not seen in a multicenter study, with only 9% of patients with a degree of clinical recovery sufficient enough to allow explantation.4
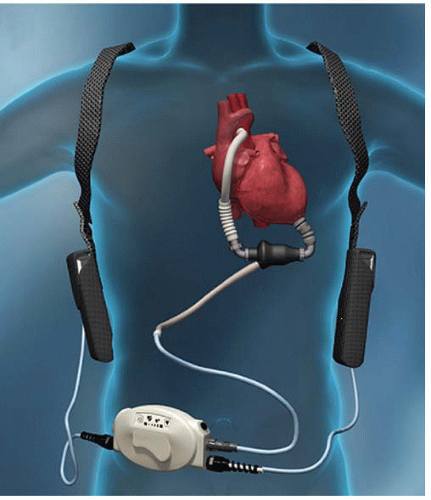
An LVAD system consists of many components (Figure 13.1). The inflow cannula is inserted into the apex of the left ventricle (LV) and blood is removed from the LV and is pumped through the LVAD and into the outflow cannula to be returned to the aorta. The LVAD pump is positioned below the left rectus abdominis muscle or in the peritoneal cavity. A percutaneous driveline connected to the LVAD pump is tunneled outside the body and is connected to an electronic controller worn on the patient’s belt. The controller is then connected to battery packs that the patient wears in a holster. Alternatively, the controller may be connected to a power base unit when the patient is at home or in the hospital.
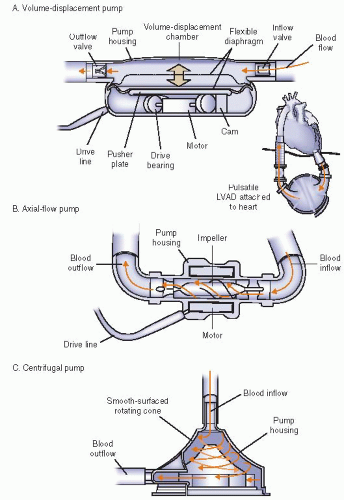
There are several different types of LVADs. First generation LVADs were pulsatile-flow devices (Figure 13.2A). These pumps have a volume displacement chamber that fills and empties with blood every time a pusher plate moves up and down. One-way valves in the inflow and outflow cannulas assure that blood is moved from the LV into the volume displacement chamber and then pumped into the aorta. Pulsatile-flow LVADs thus operate similarly to the systolic and diastolic phases of the heart and generate a systolic and diastolic blood pressure. The Thoratec Heartmate XVE (Thoratec, Pleasanton, CA) and the WorldHeart Novacor (WorldHeart, Oakland, CA) are examples of implantable pulsatile-flow LVADs. For patients too small to allow implantation of an LVAD, the Thoratec PVAD is a pulsatile-flow paracorporeal LVAD. Because of wear in the bearings and failure of the inflow valves and motor, the pulsatile-flow LVADs have a finite lifespan, usually <2 years.5 This led to the development of continuous-flow LVADs that are more durable because of fewer moving parts. There are two types of continuous-flow LVADs: axial-flow and centrifugal-flow. Axial-flow LVADs (Figure 13.2B), such as the Thoratec Heartmate II, have
an impeller that rotates around a central shaft. Blood is continuously drawn from the inflow cannula into the LVAD and then into the outflow cannula. The only moving part is the impeller. Centrifugal-flow LVADs (Figure 13.2C) have blood exit the inflow cannula at the top of the cone. Continuous spinning of the rotor causes centrifugal force, which pulls blood to the base and propels the blood into the outflow cannula. Examples of centrifugal-flow pumps are the external Thoratec CentriMag or the internal HeartWare LVAD (HeartWare International, Framingham, MA). Because of the continuous flow generated by the axial-flow and centrifugal-flow LVADs, the patient no longer has a systolic or diastolic blood pressure and pulses are no longer palpable. The ramifications of continuous-flow physiology on end-organ function will be discussed later.
an impeller that rotates around a central shaft. Blood is continuously drawn from the inflow cannula into the LVAD and then into the outflow cannula. The only moving part is the impeller. Centrifugal-flow LVADs (Figure 13.2C) have blood exit the inflow cannula at the top of the cone. Continuous spinning of the rotor causes centrifugal force, which pulls blood to the base and propels the blood into the outflow cannula. Examples of centrifugal-flow pumps are the external Thoratec CentriMag or the internal HeartWare LVAD (HeartWare International, Framingham, MA). Because of the continuous flow generated by the axial-flow and centrifugal-flow LVADs, the patient no longer has a systolic or diastolic blood pressure and pulses are no longer palpable. The ramifications of continuous-flow physiology on end-organ function will be discussed later.
CLINICAL TRIALS
The first trials looking at mechanical circulatory support with LVADs examined the BTT population. In a multicenter study of 280 patients awaiting cardiac transplantation who were unresponsive to intra-aortic balloon pump counterpulsation and inotropes, Frazier et al.6 implanted the Heartmate VE pulsatile-flow LVAD (the forerunner of the Heartmate XVE). Average cardiac flow (expressed as pump index) improved to 2.8 L/minute/m2 from a baseline cardiac index of 1.68 L/minute/m2 (P = .0001). Creatinine improved from 1.5 mg per dl at baseline to 1.1 mg per dl with LVAD support (P = .0001). Sixty-seven percent of patients were successfully bridged to cardiac transplantation compared to a control group without LVAD implantation where only 33% of patients survived to cardiac transplantation. By log-rank analysis, the probability of survival to cardiac transplantation was higher for LVAD-treated patients than for control patients (P < .001) (Figure 13.3). Average duration of LVAD therapy lasted 112 days with 54 patients being supported for >180 days. This trial was also notable because it allowed patients who were being bridged to transplantation to be released from the hospital and await their cardiac transplant at home on LVAD support.
The use of LVADs as DT was evaluated in the landmark Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) study.5 Patients with New York Heart Association (NYHA) class IV CHF with contraindications to cardiac transplantation were randomized to receive a Heartmate VE LVAD or optimal medical therapy. The primary endpoint was death from any cause. As might be expected from a trial involving patients with end-stage CHF who were ineligible for transplantation, the patient population was very ill. The average age was 68 years with an average left ventricular ejection fraction of 17% and an average blood pressure of only 102/62 mm Hg. Average cardiac index was 2 L/minute/m2, average creatinine was 1.8 mg per dl, and 69% of patients needed intravenous inotropes. Survival at 1 year was
52% in the LVAD-treated group and 25% in the medical therapy group (P = .002) (Figure 13.4). Relative risk reduction at 1 year was 48% for LVAD-treated patients and the absolute risk reduction of LVAD treatment was 27%. Survival declined at 2 years to 23% in the LVAD-treated group and to only 8% in the medical therapy group (P = .09). Cause of death was nearly universally LV failure in the medically treated group, but most commonly was sepsis or LVAD failure in the LVAD therapy group. Median NYHA class at 1 year for the LVAD group was NYHA class II and for the medical therapy group was NYHA class IV (P < .001). Although LVAD therapy afforded patients ineligible for cardiac transplantation an impressive survival benefit, the morbidity and mortality associated with the use of the pulsatile-flow LVAD was not inconsequential. Infection was a significant problem, particularly because of the relatively large percutaneous driveline. The rate of neurologic events in the LVAD-treated group was four times that of the medically treated group. Breakdown of the pulsatile flow LVAD owing to inflow valve regurgitation, erosion of the outflow graft, and wear of the motor and of the bearings were common, with a 2-year probability of device failure of 35%. Ten patients required device replacement. The only other published trial evaluating pulsatile-flow LVADs for DT was the nonrandomized study of the Novacor LVAD.7 This trial also showed a survival advantage for LVAD-treated patients with end-stage CHF compared with medically managed patients. Similar to REMATCH, this trial showed high adverse events rates associated with LVAD therapy.
52% in the LVAD-treated group and 25% in the medical therapy group (P = .002) (Figure 13.4). Relative risk reduction at 1 year was 48% for LVAD-treated patients and the absolute risk reduction of LVAD treatment was 27%. Survival declined at 2 years to 23% in the LVAD-treated group and to only 8% in the medical therapy group (P = .09). Cause of death was nearly universally LV failure in the medically treated group, but most commonly was sepsis or LVAD failure in the LVAD therapy group. Median NYHA class at 1 year for the LVAD group was NYHA class II and for the medical therapy group was NYHA class IV (P < .001). Although LVAD therapy afforded patients ineligible for cardiac transplantation an impressive survival benefit, the morbidity and mortality associated with the use of the pulsatile-flow LVAD was not inconsequential. Infection was a significant problem, particularly because of the relatively large percutaneous driveline. The rate of neurologic events in the LVAD-treated group was four times that of the medically treated group. Breakdown of the pulsatile flow LVAD owing to inflow valve regurgitation, erosion of the outflow graft, and wear of the motor and of the bearings were common, with a 2-year probability of device failure of 35%. Ten patients required device replacement. The only other published trial evaluating pulsatile-flow LVADs for DT was the nonrandomized study of the Novacor LVAD.7 This trial also showed a survival advantage for LVAD-treated patients with end-stage CHF compared with medically managed patients. Similar to REMATCH, this trial showed high adverse events rates associated with LVAD therapy.
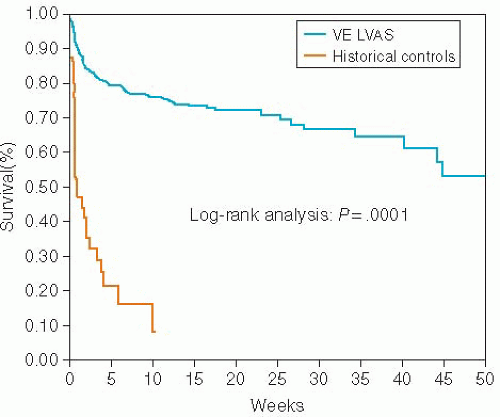 Figure 13.3. Probability of survival to transplantation for HeartMate VE LVAD-treated patients versus historical control patients by log-rank analysis. |
Although the Heartmate XVE was approved for DT in the United States by the Food and Drug Administration (FDA) in November 2002, only 451 patients received an LVAD as DT during the first 5 years after the REMATCH trial.8 For greater adoption of mechanical circulatory support technology for the DT patients, improvements needed to be made in the durability and adverse event profile of LVADs. These improvements came in the form of the axial-flow LVADs. Axial-flow LVADs such as the Heartmate II have only one moving part (the impeller), and because blood is constantly being suctioned from the LV cavity and ejected into the aorta, there is no need for valves (which can fail) in the inflow and outflow cannulas. The driveline of the Heartmate II is smaller than that of the Heartmate XVE. The Heartmate II pump itself is much smaller than the Heartmate XVE pump (0.39 kg and 63 ml vs. 1.25 kg and 450 ml) (Figure 13.5). Before the introduction of the Heartmate II, implantable LVADs were limited to patients with a body surface area (BSA) of >1.5 m2, restricting the use of this therapy in women and adolescents. The only option for patients with smaller BSAs was to have a paracorporeal LVAD such as the Thoratec PVAD. Furthermore, the newer continuous-flow pumps are much quieter than the pulsatile-flow LVADs.
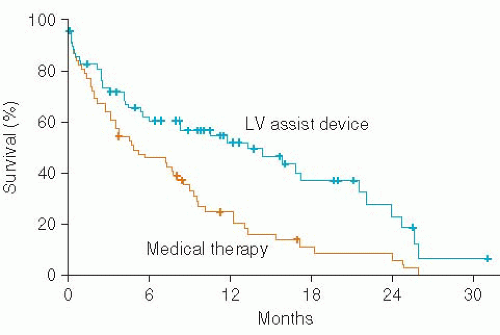 Figure 13.4. Kaplan-Meier curves for survival of patients receiving a pulsatile flow LVAD (Heartmate VE) versus optimal medical therapy. |
Before testing the axial-flow technology in the long-term use DT setting, the devices were tested in the BTT population. In a prospective multicenter observational study, the Heartmate II was implanted in 133 patients awaiting cardiac transplantation with a primary endpoint of cardiac transplantation, cardiac recovery allowing device explantation, or continued mechanical support at 180 days and remaining eligible for cardiac transplant. Twenty-four hours after device implantation, cardiac index increased from 2.0 to 2.8 L/minute/m2 (P < .001), whereas pulmonary capillary wedge pressure (PCWP) decreased from 26 to 16 mm Hg (P < .001) and mean pulmonary artery pressure (PAP) decreased from 37 to 26 mm Hg (P < .001). Although 100% of patients at enrollment into the study had NYHA class IV symptoms, after 3 months of continuous-flow support, only 3% of patients had NYHA class IV symptoms with 83% having NYHA classes I-II symptoms. From the 133 patients studied, 100 patients (75%) met the primary endpoint, with 56 patients being successfully bridged to cardiac transplantation, 1 patient having cardiac recovery with device explantation, and 43 patients continuing to receive mechanical circulatory support while awaiting cardiac transplantation. The commonest adverse effects were bleeding, ventricular arrhythmias, localized infections not related to the LVAD, and sepsis. A follow-up study by the same investigators reported data on the first 281 patients enrolled who had reached the clinical endpoints or had 18 months of follow-up after Heartmate II implantation.9 Seventy-nine percent of patients received a cardiac transplant, had device explantation owing
to cardiac recovery, or were still alive on LVAD support with 18 months follow-up. Overall survival was 82% at 6 months, 73% at 1 year, and 72% at 18 months. The longest duration of support in the study was 3.1 years with a median duration of 1.6 years. Questions arose about the effects of this degree of long-term mechanical circulatory support on end-organ function as the kidney and liver previously received pulsatile flow from the heart in vivo. Russell et al.10 were able to demonstrate that with continuous-flow LVAD support, patients with normal blood urea nitrogen (BUN) and creatinine and aspartate transaminase (AST), alanine transaminase (ALT), and total bilirubin remained with normal values of these parameters of renal and hepatic function during LVAD support. For patients with abnormal values of renal and liver function, after 6 months of continuous-flow LVAD therapy, BUN improved from 37 to 23 mg per dl (P < .0001) and creatinine decreased from 1.8 to 1.4 (P < .01). AST and ALT decreased from 121 and 171 international units to 36 and 31 international units (P < .001), respectively. Total bilirubin also decreased from 2.1 to 0.9 mg per dl (P < .0001). Thus, long-term support with a continuous-flow LVAD was effective at providing hemodynamic support while improving end-organ perfusion in patients awaiting cardiac transplantation.
to cardiac recovery, or were still alive on LVAD support with 18 months follow-up. Overall survival was 82% at 6 months, 73% at 1 year, and 72% at 18 months. The longest duration of support in the study was 3.1 years with a median duration of 1.6 years. Questions arose about the effects of this degree of long-term mechanical circulatory support on end-organ function as the kidney and liver previously received pulsatile flow from the heart in vivo. Russell et al.10 were able to demonstrate that with continuous-flow LVAD support, patients with normal blood urea nitrogen (BUN) and creatinine and aspartate transaminase (AST), alanine transaminase (ALT), and total bilirubin remained with normal values of these parameters of renal and hepatic function during LVAD support. For patients with abnormal values of renal and liver function, after 6 months of continuous-flow LVAD therapy, BUN improved from 37 to 23 mg per dl (P < .0001) and creatinine decreased from 1.8 to 1.4 (P < .01). AST and ALT decreased from 121 and 171 international units to 36 and 31 international units (P < .001), respectively. Total bilirubin also decreased from 2.1 to 0.9 mg per dl (P < .0001). Thus, long-term support with a continuous-flow LVAD was effective at providing hemodynamic support while improving end-organ perfusion in patients awaiting cardiac transplantation.
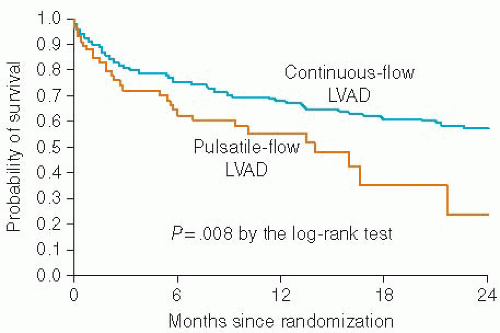
The Heartmate II continuous-flow LVAD was compared with the pulsatile-flow Heartmate XVE for DT in a randomized multicenter trial that enrolled 200 patients.11 The primary endpoint was a composite of survival at 2 years free of a disabling stroke or reoperation to replace the LVAD. The average age of the patients was 62 years and the average LV EF was 17%. Eighty percent of patients were on intravenous inotropes and 60% of patients had a cardiac resynchronization device. Twenty-two percent of patients were on intra-aortic balloon pump support prior to LVAD placement. Forty-six percent of patients with a continuous-flow LVAD reached the primary endpoint compared to 11% of patient with a pulsatile flow LVAD (P < .001). Of the individual components of the primary endpoint, the hazard ratio (HR) for a disabling stroke was similar between the continuous-flow and the pulsatile-flow LVAD (HR 0.78, P = .56). The HRs for reoperation to repair or replace the LVAD (HR 0.18, P < .001) and death within 2 years after LVAD implantation (HR 0.59, P = .048) significantly favored the continuous-flow LVAD. Of the 59 patients who received a pulsatile-flow LVAD, 20 required 21 pump replacements. Of the 133 patients who received a continuous-flow LVAD, only 12 required 13 pump replacements. By as-treated analysis, actuarial survival was improved for patients with a continuous-flow LVAD (1- and 2-year survival rates of 68% and 58%, respectively) compared with the pulsatile-flow LVAD (1- and 2-year survival rates of 55% and 24%, respectively) (P = .008) (Figure 13.6). The 1- and 2-year survival rates for the pulsatile-flow patients were identical to the 1- and 2-year survival rates from the REMATCH study.5 Despite improved experience, surgical technique, and postoperative care, the survival of the pulsatile-flow LVAD group could not be improved upon over the course of a decade. Rates of device-related infection, sepsis, right heart failure (RHF), respiratory failure, renal failure, and cardiac arrhythmia were all lower in the continuous-flow LVAD-treated group.11 The rates of stroke and bleeding did not differ between the continuous-flow LVAD-treated group and the pulsatile-flow LVAD group. Based on the results of this study the HeartMate II was approved for DT by the FDA.12
COMPLICATIONS
As experience with mechanical circulatory support has been obtained, several common complications have been noted.
INFECTIONS
Sepsis was the commonest cause of death (41%) in the LVAD-treated patients in the REMATCH study.5 Prevention of sepsis and other infections begins prior to LVAD insertion. Preoperative bathing of patients with an antiseptic, rotation and removal of intravenous lines, dental hygiene, eradication of Staph aureus




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree