High-risk surgical patients undergoing transcatheter aortic valve implantation (TAVI) represent an emerging population, which may benefit from short-term use of mechanical circulatory support (MCS) devices. The aim of this study was to determine the practice and inhospital outcomes of MCS utilization in patients undergoing TAVI. We analyzed data from Nationwide Inpatient Sample (2011 and 2012) using the International Classification of Diseases, Ninth Revision, Clinical Modification procedure codes . A total of 1,794 TAVI procedures (375 hospitals in the United States) were identified of which 190 (10.6%) used an MCS device (MCS group) and 1,604 (89.4%) did not (non-MCS group). The use of MCS devices with TAVI was associated with significant increase in the inhospital mortality (14.9% vs 3.5%, p <0.01). The mean length (11.8 ± 0.8 vs 8.1 ± 0.2 days, p <0.01) and cost ($68,997 ± 3,656 vs $55,878 ± 653, p = 0.03) of hospitalization were also significantly greater in the MCS group. Ventricular fibrillation arrest, transapical access for TAVI, and cardiogenic shock were the most significant predictors of MCS use during TAVI. In the multivariate model, use of any MCS device was found to be an independent predictor of increased mortality (odds ratio 3.5, 95% confidence interval 2.6 to 4.6, p <0.0001) and complications (odds ratio 3.3, 95% confidence interval 2.8 to 3.9, p <0.0001). The propensity score–matched analysis also showed a similar result. In conclusion, the unacceptably high rates of mortality and complications coupled with a significant increase in the length and cost of hospitalization should raise concerns about utility of MCS devices during TAVI in this prohibitive surgical risk population.
Use of mechanical circulatory support (MCS) devices in cardiogenic shock (CS), acute myocardial infarction (AMI), high-risk percutaneous coronary interventions (PCI) has been extensively studied. High-risk surgical patients with valvular heart diseases undergoing percutaneous treatment such as transcatheter aortic valve implantation (TAVI) represent an emerging population, which may benefit from short-term use of MCS. The pivotal Placement of Aortic Transcatheter Valve (PARTNER) trial excluded patients with severe left ventricular dysfunction (left ventricular ejection fraction <20%), bulky calcified aortic valve leaflets in close proximity to coronary ostia, hemodynamic instability requiring inotropic therapy or MCS devices, and recent myocardial infarction (<1 month) or untreated coronary disease. However, in clinical practice, after regulatory approval of TAVI in the United States (US), MCS may be used preemptively in such patients presenting with exceedingly high periprocedural risk or emergently for patients with procedural complications of TAVI. The aim of this study was to determine the practice and inhospital outcomes of MCS utilization with TAVI in an unrestricted patient population using the nation’s largest hospitalization database.
Methods
Data were obtained from the Nationwide Inpatient Sample (NIS), which is a part of a family of databases developed for the Healthcare Cost and Utilization Project and is sponsored by the Agency for Healthcare Research and Quality (AHRQ). The study involved subjects from deidentified database, so it was exempted from institutional review board’s approval. Data from the NIS have previously been used to identify, track, and analyze national trends in health care usage, patterns of major procedures, access, disparity of care, trends in hospitalizations, charges, quality, and outcomes. We analyzed data from 2011 to 2012 using the International Classification of Diseases, Ninth Revision, Clinical Modification procedure codes for TAVI ( Figure 1 ). The use of MCS was identified by their respective International Classification of Diseases, Ninth Revision, Clinical Modification codes, that is, 37.61 for intra-aortic balloon pump (IABP), 37.68 and 37.62 for percutaneous ventricular assist devices (PVADs) including Impella and TandemHeart, 39.65 and 39.66 for extracorporeal membrane oxygenation (ECMO) and percutaneous cardiopulmonary support, and 39.61 for cardiopulmonary bypass (CBP). We excluded cases with missing baseline or outcome information, and the remaining TAVI cases were divided into 2 categories on the basis of utilization of MCS.
We defined severity of co-morbid conditions using Deyo modification of Charlson Comorbidity Index (CCI). Procedural complications were identified by Patient Safety Indicators, version 4.4, March 2012, which have been established by the AHRQ to monitor preventable adverse events during hospitalization. Other outcomes studied were the length of hospital stay and cost of hospitalization. To estimate the cost of hospitalization, the NIS data were merged with cost-to-charge ratios available from the Healthcare Cost and Utilization Project. Cost for each year was calculated in terms of the 2012 cost, after adjusting for inflation according to the latest consumer price index data released by the US government on January 16, 2013. This method of identifying patients undergoing procedures, co-morbid conditions, and associated outcomes in terms of mortality, complications, length of hospital stay, and cost of hospitalization has previously been used in several studies.
Stata IC 11.0 (StataCorp, College Station, Texas) and SAS 9.3 (SAS Institute Inc., Cary, North Carolina) were used for analyses, which accounted for the complex survey design and clustering. Multivariate simple logistic regression models (with patient-level factors nested within hospital-level factors) were generated to identify the independent predictors of inhospital mortality and procedural complications. All interactions were thoroughly tested. Collinearity was assessed using variance inflation factor.
We used propensity scoring method to establish matched cohorts to control for imbalances of patients’ and hospitals’ characteristics between the 2 groups, which may have influenced the primary outcome. A propensity score was assigned to each hospitalization, and the following variables were adjusted for: age, gender, Charlson co-morbidity score, AMI, CS, cardiac arrest, type of admission, TAVI access, and primary payer. Patients with similar propensity score in the 2 treatment groups were matched using a one-to-one scheme without replacement using greedy algorithm.
Results
A total of 1,794 TAVI procedures (which translates to an estimated 8,828 procedures performed in 375 hospitals in the US) were identified of which 190 (10.6%) used an MCS device (MCS group) and 1,604 (89.4%) did not (non-MCS group). Table 1 demonstrates the baseline characteristics of the study population. There were significant differences between the baseline characteristics of patients in the 2 groups ( Table 1 ). A higher percentage of patients in MCS group underwent transapical TAVI (54% vs 13%, p <0.01), had AMI (6.4% vs 2.1%, p <0.01), underwent PCI (5.4% vs 2.1%, p <0.01), had cardiac arrest (10% vs 2.3%, p ≤0.01; including ventricular fibrillation 8% vs 1%, p ≤0.01), and CS (16.8% vs 2.9%, p <0.01) compared to the non-MCS group. In contrast, the patients in non-MCS group were older (71% vs 37% aged >80 years, p <0.01) and had a greater mean Charlson co-morbidity score (2.65 ± 0.04 vs 2 ± 0.1, p <0.01). A significantly greater percentage of patients in the non-MCS group had congestive heart failure, chronic obstructive pulmonary disease, and peripheral arterial disease (p value for all <0.01).
| (2011-2012) | TAVI with Hemodynamic Support | |||
|---|---|---|---|---|
| Variable | No n= 1604 | Yes n=190 | Overall | P-value |
| Total No. Of Observations (unweighted) | 1604 (89.41%) | 190 (10.59%) | 1794 | |
| Total No. Of Observations (weighted) | 7893 (89.41%) | 935 (10.59%) | 8828 | |
| No. Of Hospitals (Unweighted) | 248 (66.13%) | 127 (33.87%) | 375 | |
| Age (Years) | ||||
| Mean +/- SE | 82.0 (0.20) | 74.1 (0.91) | 81.2 (0.21) | <0.01 |
| 18 -49 | 0.2% | 5.4% | 0.7% | |
| 50-59 | 1.6% | 7.5% | 2.2% | |
| 60-69 | 6.6% | 18.8% | 7.9% | <0.01 |
| 70-79 | 20.6% | 31.1% | 21.7% | |
| >80 | 71.1% | 37.3% | 67.5% | |
| Male | 51.7% | 52.0% | 51.8% | 0.9036 |
| Female | 48.3% | 48.1% | 48.2% | |
| White | 78.9% | 75.2% | 78.5% | |
| Black | 3.7% | 1.6% | 3.5% | <0.01 |
| Hispanic | 3.4% | 10.2% | 4.1% | |
| Other | 8.3% | 4.7% | 7.9% | |
| Missing | 5.7% | 8.3% | 6.0% | |
| Comorbidities | ||||
| Charlson Score | ||||
| Mean +/- SE | 2.7 (0.04%) | 2.0 (0.12%) | 2.6 (0.04%) | <0.01 |
| 0 | 6.8% | 18.4% | 8.0% | |
| 1 | 21.2% | 25.3% | 21.6% | <0.01 |
| More than or equal to 2 | 72.1% | 56.3% | 70.4% | |
| Obesity ∗ | 13.2% | 14.3% | 13.3% | 0.3515 |
| Hypertension | 80.4% | 77.7% | 80.2% | 0.0468 |
| Diabetes Mellitus | 33.4 | 30.9 | 33.2% | 0.1173 |
| Acute Myocardial Infarction | 2.1% | 6.4% | 2.6% | <0.01 |
| Heart Failure | 72.6% | 54.5% | 70.7% | <0.01 |
| Chronic Pulmonary Disease | 35.6% | 25.7% | 34.6% | <0.01 |
| Peripheral Vascular Disease | 31.3% | 25.7% | 30.7% | 0.0004 |
| Fluid-electrolyte abnormalities & Renal Failure | 51.8% | 48.1% | 51.4% | 0.0297 |
| Bed size of Hospital † | ||||
| Small | 4.0% | 2.7% | 3.9% | |
| Medium | 13.1% | 12.9% | 13.1% | 0.1341 |
| Large | 82.9% | 84.4% | 83.1% | |
| Hospital Location & Teaching Status | ||||
| Rural | 0.6% | 0.0% | 0.5% | <0.01 |
| Urban Non-teaching | 9.5% | 16.0% | 10.2% | |
| Urban Teaching | 90.0% | 84.0% | 89.3% | |
| Cardiac Arrest | 2.3% | 10.0% | 3.1% | <0.01 |
| Ventricular Fibrillation | 1.0% | 7.9% | 1.7% | <0.01 |
| Use of Vasopressor Agents | 2.4% | 1.6% | 2.3% | 0.1354 |
| Cardiopulmonary Resuscitation | 1.7% | 7.9% | 2.3% | <0.01 |
| Percutaneous Coronary Intervention | 2.1% | 5.4% | 2.5% | <0.01 |
| Cardiogenic Shock | 2.9% | 16.7% | 4.3% | <0.01 |
| Type of TAVI Access | ||||
| Transfemoral | 87.0% | 46.0% | 82.7% | <0.01 |
| Transapical | 13.0% | 54.0% | 17.4% | |
∗ Obesity defined as https://www.hcup-us.ahrq.gov/db/vars/cm_obese/nisnote.jsp#top .
† Hospitals were categorized into small, medium, and large based on the bed size of the hospital on the basis of hospital’s location and teaching status. http://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nisnote.jsp .
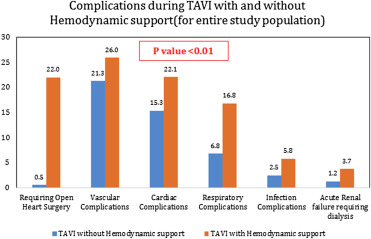
The use of MCS devices with TAVI was associated with over 325% increase in the inhospital mortality rate (14.9% vs 3.5%, p <0.01), and 70% of the patients in the MCS group experienced at least 1 procedural complication compared to 40% in non-MCS group ( Table 2 ). The mean length (11.8 ± 0.8 vs 8.1 ± 0.2 days, p <0.01) and cost ($68,997 ± 3,656 vs $55,878 ± 653, p = 0.03) of hospitalization were also significantly greater in MCS group. The rates of cardiac, respiratory, renal, and vascular complications were significantly higher, and 22% patients required open heart surgery in MCS group ( Figure 2 ). CPB (71.6%, n = 136) was the most frequently used MCS device followed by IABP (18.9%, n = 36), ECMO (7.4%, n = 14), and PVADs (2.1%, n = 4). The outcomes associated with individual devices are provided in Supplementary Table 1 . Female gender, AMI, concomitant PCI, CS, ventricular fibrillation arrest, and transapical access for TAVI were significant predictors of MCS use during TAVI ( Table 3 ).
| Outcomes | TAVI without Hemodynamic support | TAVI with Hemodynamic support | Overall | P-value |
|---|---|---|---|---|
| Death | 3.5% | 14.9% | 4.7% | <0.01 |
| Any complication | 40.3% | 69.9% | 43.4% | <0.01 |
| Death + Any complications | 41.2% | 71.0% | 44.3% | <0.01 |
| Length of Stay (Mean, SE) | 8.1 (0.16%) | 11.8 (0.78%) | 8.5 (0.16%) | <0.01 |
| Length of hospital-stay – Median (Quartile 1, 3), days | 6 (4, 10) | 8 (5, 15) | 6 (4, 10) | |
| Cost ($) (Mean, SE) | 55,878 (653) | 68,997 (3656) | 57292 (709) | 0.0325 |
| Multivariate Simple logistic regression for use of Hemodynamic support devices (Weighted) | ||||
|---|---|---|---|---|
| TAVI and hemodynamic support (2011-2012) | Unmatched Dataset | |||
| Variable | Odds Ratio (95% CI) | LL | UL | P-Value |
| Age (in 5 year increment) | 0.68 | 0.65 | 0.71 | <.0001 |
| Sex | ||||
| Male | 1 | Ref | Ref | Ref |
| Female | 1.25 | 1.05 | 1.48 | 0.01 |
| Charlson Score | ||||
| 0 | 1 | Ref | Ref | Ref |
| 1 | 0.55 | 0.42 | 0.73 | <.0001 |
| More than or equal to 2 | 0.29 | 0.22 | 0.37 | <.0001 |
| Acute Myocardial Infarction | 3.17 | 2.13 | 4.71 | <.0001 |
| Cardiogenic Shock | 9.21 | 6.9 | 12.3 | <.0001 |
| Cardiac Arrest | 2.84 | 1.97 | 4.08 | <.0001 |
| Ventricular Fibrillation | 18.45 | 11.98 | 28.43 | <.0001 |
| Use of Vasopressor Agents | 0.18 | 0.09 | 0.37 | <.0001 |
| Percutaneous Coronary Intervention | 4.14 | 2.81 | 6.1 | <.0001 |
| Type of Access | ||||
| Transfemoral | 1 | Ref | Ref | Ref |
| Transapical | 9.69 | 8.14 | 11.55 | <.0001 |
| Hospital Region | ||||
| Northeast | 1 | Ref | Ref | Ref |
| Midwest | 1.35 | 1.02 | 1.79 | 0.037 |
| South | 2.73 | 2.15 | 3.47 | <.0001 |
| West | 2.04 | 1.54 | 2.71 | <.0001 |
In the multivariate model, use of any MCS device was found to be an independent predictor of increased mortality (odds ratio 3.5, 95% confidence interval [CI] 2.6 to 4.6, p <0.0001) and complications (odds ratio 3.3, 95% CI 2.8 to 3.9, p <0.0001; Table 4 ). Other predictors of increased mortality were older age, female gender, CS, cardiac arrest, use of vasopressor agents, and performing PCI. Predictors of increased complications are listed in Supplementary Table 2 . The propensity score–matched analysis is provided in Supplementary Table 3 . In this analysis, multiple patient- and hospital-level variables which could have affected the outcome were adjusted for. The propensity score–matched analysis (160 patients in each group) also showed a significant increase in the inhospital mortality (15.3% vs 9%, p <0.01), length, and cost of hospitalization associated with the use of MCS devices in TAVI ( Table 5 ).
| TAVI and hemodynamic support (2011-2012) | Unmatched Dataset | |||
|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) | LL | UL | P-Value |
| Use of Hemodynamic Support | ||||
| No Use | 1 | Ref | Ref | Ref |
| Use of Hemodynamic Support | 3.45 | 2.57 | 4.61 | <0.01 |
| Age (in 5 year increment) | 1.29 | 1.20 | 1.38 | <0.01 |
| Sex | ||||
| Male | 1.00 | Ref | Ref | Ref |
| Female | 1.27 | 1.01 | 1.59 | 0.0383 |
| Charlson Score | ||||
| 0 | 1.00 | Ref | Ref | Ref |
| 1 | 0.71 | 0.44 | 1.16 | 0.1741 |
| More than or equal to 2 | 1.05 | 0.68 | 1.62 | 0.8382 |
| Acute Myocardial Infarction | 1.68 | 0.99 | 2.87 | 0.0562 |
| Cardiogenic Shock | 7.38 | 5.46 | 9.97 | <0.01 |
| Cardiac Arrest | 6.86 | 4.88 | 9.64 | <0.01 |
| Use of Vasopressor Agents | 7.18 | 4.88 | 10.55 | <0.01 |
| Percutaneous Coronary Intervention | 2.15 | 1.32 | 3.51 | 0.0023 |
| Bed size of Hospital depending on Location & Teaching Status ∗ | ||||
| Small | 1.00 | Ref | Ref | Ref |
| Medium | 0.80 | 0.49 | 1.31 | 0.3796 |
| Large | 0.41 | 0.26 | 0.63 | <0.01 |
| Admission Day | ||||
| Weekdays | 1.00 | Ref | Ref | Ref |
| Weekends | 0.48 | 0.29 | 0.81 | 0.0058 |
| Hospital Region (%) | ||||
| Northeast | 1.00 | Ref | Ref | Ref |
| Midwest | 1.59 | 1.13 | 2.23 | 0.0074 |
| South | 1.25 | 0.91 | 1.72 | 0.1733 |
| West | 0.97 | 0.66 | 1.42 | 0.8764 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


