Mastery of Endovascular Surgical Treatment of Abdominal Aortic Aneurysms
R. J. Hinchliffe
B. R. Hopkinson
The endovascular technique for repair of abdominal aortic aneurysms (AAA) was first described in 1991. Although the endovascular aneurysm repair (EVAR) approach differs from open repair, both procedures aim to prevent death from rupture. Passing blood from the normal artery above to the normal artery below prevents the aneurysm from rupturing.
Endovascular procedures were once seen as the remit of the radiologist. With the advent of EVAR, the field is changing, and surgeons must now be fully familiar with all types of endovascular procedures, their indications, contraindications, and complications. If nothing else, a number of patients will require adjunctive surgical procedures, and at worse, they will require conversion to open repair.
Although it is a widely accepted technique, EVAR continues to be debated. The attractions of EVAR are principally due to its minimally invasive nature. The reduced physiologic impact allows patients to recover more quickly and permits more ill patients to undergo surgery.
We will discuss the Nottingham approach to EVAR and include some of the useful techniques and potential pitfalls that have been developed through experience, practice, and learning from other surgeons. This discussion is not intended as a panacea but merely a reflection on some of the salient points of EVAR. Ideally, the reader can avoid relearning the mistakes made by the authors and others. Some points have a scientific basis, while others are observations made over many hours in the operating room. The approaches described work reliably in Nottingham, but there is always more than one possible approach. Accordingly, we have included some techniques that are not used in Nottingham but have been favored by others. Detailed descriptions of these techniques are available elsewhere.
Pre-operative Preparation
Although aneurysm morphology dominates the pre-operative assessment of patients for EVAR, the general physiologic assessment and optimization of the patient must not be forgotten. Particular attention should be paid to cardiovascular, respiratory, and renal function, as aortic occlusion occurs at least briefly during all EVAR, and peri-operative complications can occur frequently. A patient’s physiologic assessment should not differ whether the intended surgical repair is open or endovascular.
Aneurysm morphology can be assessed with a variety of modalities. Early in the evolution of EVAR, calibration angiography was performed in all patients. Now that improved noninvasive imaging techniques are available, most experienced centers rely solely upon spiral computed tomographic angiography (spiral CTA) for preoperative workup. Multiplanar reformatting allows accurate assessment of aneurysm length. Any discrepancy between the assessment of length between spiral CTA and angiography is minor, and with the use of modular stent grafts is hardly clinically relevant. Consequently, calibration angiography is now reserved for complex cases (e.g., fenestrated endovascular stent graft) where extra data are valued or where intervention may be contemplated (e.g., renal artery angioplasty).
Table 18-1 Ideal Morphologic Characteristics for EVAR | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Other centers have embraced preoperative magnetic resonance (MR) angiography (MRA), but this requires significant postimage processing and cannot be used in the follow up of patients with ferrous stent grafts.
Ideal Aneurysm Morphology
The requirements are first that the anatomy permits access and delivery of the stent graft to its desired site, and second, that there is a sufficiently normal artery above and below to create a seal and fixation (Table 18-1).
With increasing experience, improving technology, and the use of adjunctive procedures, the proportion of aneurysms that are treatable by EVAR is increasing.
stent graft configuration has evolved. Straight aorto-aortic stent grafts have largely been consigned to history. They had low applicability due to the requirement of a distal aortic neck and were associated with a high incidence of distal type I endoleak. The sole indication for aorto-aortic grafts remains an isolated saccular aneurysm of the aorta. The majority of stent grafts are now bifurcated, although the uni-iliac configuration may be useful where rapid aneurysm exclusion is desirable, such as in ruptured AAA or where there are adverse features in one iliac system.
Modular stent grafts allow intra-operative customization of length, whereas unitary systems require more accurate prediction of length but have the drawback of potential endoleak at the interface between components.
Stent Planning
There are several core requirements during planning of a patient for stent graft placement. This text does not describe the attributes and drawbacks of all of the commercially available devices, but where pertinent some illustrations may be included.
Oversizing of stent graft diameter (with respect to native arterial external diameter) reduces the incidence of endoleak. When planning, stents should be oversized in the region of 2 to 4 mm proximally and 1 to 2 mm distally.
Predicting stent graft length is notoriously difficult. This is mainly because it is not always possible to anticipate how a stent graft will lie in vivo, particularly within a large, empty aneurysm sac. stent grafts will not invariably adopt the lie of the calibration angiographic catheter or the curvilinear reformat. Modular stent grafts with generous overlap between components allow for any length discrepancy. The intra-operaive lengths invariably appear to be longer than predicted from pre-operative imaging.
Stent Graft Configuration
The forces generated in the aorta by blood flow will exert in the region of 10N on any given device. When considering any system, thought must be given to fixation to prevent dislodgement by this force. Fixation is currently brought about by radial force (from either self-expanding or balloon expandable stents), hooks, or barbs, or by more than one these three. The contribution of columnar strength from a rigid device remains controversial. The use of suprarenal fixation systems has facilitated stent graft deployment in shorter necks; concerns thus far over renal artery embolization have largely been unfounded.
Deployment of a Stent Graft
Before embarking upon EVAR it is important to have anesthetic and nursing staff who have some familiarity with the technique. The nursing staff needs to be fully conversant with the names and assimilation of the endovascular kit. The anesthetic staff should be familiar with positioning of patients for the operation and surgical exposure required. Whether the procedure is performed in the operating room or an interventional radiology suite is probably of little consequence as long as there is a good image intensifier in the operating room or adequate infection control in radiology.
Before embarking on any particular EVAR, the surgeon should have available a stock of extra guidewires, sheaths, catheters, and stent grafts so that they will be available in an emergency. Availability of these extras may make the difference between a successful EVAR and conversion to open repair. Consequently, many stent graft manufacturers provide a supplementary kit.
The patient in Nottingham undergoes EVAR in an operating room under epidural anesthesia. Enthusiasts have demonstrated the feasibility of EVAR under local anesthesia, but epidural anesthesia is tolerated well by the majority of patients and helps the patient to be comfortable and still.
The patient is placed supine on an operating table with angiographic tunnel and prepared with an antiseptic solution. The positioning should permit access to both groins and abdomen. All patients receive antibiotic prophylaxis, and heparin is given systemically prior to graft insertion. The groin incision should facilitate access to the common femoral arteries (CFA) bilaterally. Control of the superficial femoral and profunda femoris arteries is not necessary. Angiographic needles are inserted into the CFA, and floppy guidewires are passed into the supraceliac aorta, being careful to avoid dissection by gentle manipulation and constant screening. Before a catheter is introduced into the artery, a sheath is placed to avoid traumatizing the arterial wall during multiple passages. Wires are cleaned with heparinized saline to reduce friction.
In Nottingham we have found the use of bilateral 4Fr angiographic catheters helpful. The radiopaque markers on the top of these 4Fr catheters sit low in the aneurysm sac. By their presence they usually demonstrate the position of the aortic bifurcation and are particularly useful when deploying bifurcated modular stent grafts. Later in the procedure they can be pulled slowly down into the common iliac artery and can be used to accurately demonstrate the site of the internal iliac arteries. They also allow last-minute adjustment of the position at the bottom end of the iliac limbs of the graft, thereby avoiding internal iliac occlusion and allowing maximum coverage of the common iliac down to the bifurcation.
Stiff guidewires are essential to the successful insertion of the graft carrier. They facilitate insertion by straightening out the iliac arteries and preventing kinks. During early experiences with EVAR a number of patients had to be converted to open repair or were turned down for EVAR because of the presence of tortuous iliac arteries. Iliac tortuousity alone is usually a surmountable problem with stiff wires but in association with severe calcification may prevent device insertion. Stiff wires should always be inserted through a catheter and never advanced alone because of the potential arterial damage that they may cause.
Orientation of the stent graft is performed outside the patient. In particular, the surgeon is looking for the site of radiopaque markers, which are often found on the iliac limbs, stumps, and so on. It is always necessary to make sure the graft is actually loaded onto the graft carrier and contains the required number of stents.
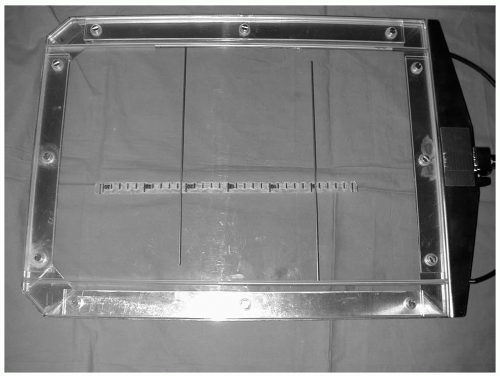
Before device insertion the renal arteries are localized by screening to the T12-L1 vertebral level. Exact site is confirmed by calibration angiography. Contrast injections are ideally performed using a power injector. However, satisfactory images can be obtained by hand injections through 7Fr catheters. In Nottingham, a marking device is used to mark the position of the renal arteries (Fig. 18-1.). Screening up and down the patient while inserting the stent graft can result in loss of the location. The system comprises mobile radiopaque markers in the angiographic tunnel, the calibration angiographic catheter in the aorta, and a marker on the C-arm. When the marker in the angiographic tunnel and the calibration catheter are fixed, it is possible to move the C-arm up and down the
patient (e.g., to observe stent graft delivery) and to come back to exactly the same position. This technique avoids parallax errors and reduces contrast volume and operative time.
patient (e.g., to observe stent graft delivery) and to come back to exactly the same position. This technique avoids parallax errors and reduces contrast volume and operative time.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree