Management of Infected Aortic Grafts
Thomas S. Huber
Infected aortic grafts represent one of the most difficult problems faced by peripheral vascular surgeons, and appropriate management frequently requires some creativity. Fortunately, the overall incidence is quite low. The treatment objectives include control of any underlying sepsis, removal of the infected graft material, revascularization of the torso/lower extremities, and control of the bleeding in the case of an aortoenteric fistula (AEF). The majority and most significant graft infections involve the infrarenal aorta, and their management will be the focus of this chapter. The treatment options for infected suprarenal/thoracoabdominal aortic grafts are limited and usually necessitate in situ replacement.
Diagnosis
Patients with infected aortic grafts may present anywhere along the spectrum from nonspecific complaints to overwhelming sepsis. The diagnosis is simplified in the presence of a draining sinus tract and/or an exposed graft, although this presentation is fairly unusual. Indeed, only 5% of the patients have positive blood cultures at the time of presentation. The majority of patients present with nonspecific symptoms, including a low-grade temperature, mildly elevated leukocyte count, an elevated sedimentation rate, malaise, and a generalized “failure to thrive.” Predictably, the diagnosis can be difficult. This is not particularly surprising given the low virulence of Staphylococcus epidermidis that accounts for a significant percentage of the infections. It is imperative that once the suspicion of an infected graft is raised, the appropriate evaluation should be initiated to confirm or refute the diagnosis. The initial operative report and immediate postoperative course should be reviewed for any complicating factors. All patients with gastrointestinal bleeding and a prosthetic aortic graft should be presumed to have an AEF until proven otherwise. Notably, both femoral pseudoaneurysms and limb thromboses after aortobifemoral bypass may result from graft infections, with the latter occurring in up to 25% of the cases.
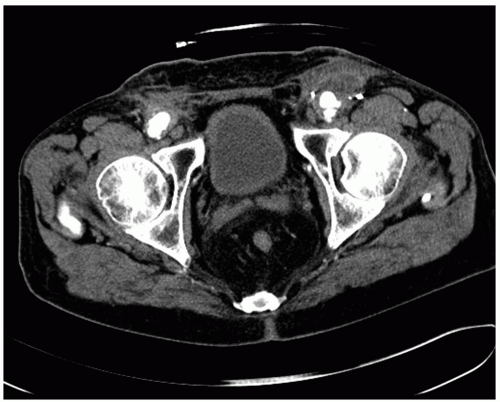
A contrast CT scan is the diagnostic study of choice with sensitivities and specificities >90% (Fig. 49-1). The specific findings suggestive of an aortic graft infection include perigraft fluid collection and/or soft tissue swelling, ectopic gas, pseudoaneurysm (aortic or femoral anastomoses), retroperitoneal abscess, bowel wall thickening, or hydroureter. Indeed, the finding of a hydroureter in the absence of other findings should suggest an infected graft and is almost pathognomonic. Notably, it is difficult to diagnose an infected graft in the early postoperative period, because many of the normal postoperative changes are similar to those seen with an infected graft; gas around the graft resolves within 2 weeks postoperatively, while fluid around the graft can persist for up to 3 months.
A variety of other imaging studies have been used to confirm the diagnosis of an infected graft. MRI may be superior to CT because of its ability to resolve differences in the soft tissues, although the overall experience is somewhat limited and most surgeons are more familiar with CT images. Ultrasound may be helpful to identify perigraft fluid, particularly in the groin, and to confirm the presence of a pseudoaneurysm. Contrast can be injected in any tract (i.e., sinogram) that courses near the graft in an attempt to determine whether there is a communication with the graft itself. Several radionuclide functional studies have been used in this setting, with indium-labeled leukocytes being the most common. Although the associated sensitivity and specificities are reasonable, all the radionuclide studies suffer from the fact that areas of inflammation can lead to false positive findings, while antibiotic therapy can lead to false negatives. Regardless, they can be helpful in equivocal cases. Arteriography has no role in the diagnosis of an infected graft, although it is routinely used for operative planning. Surgical exploration is definitive and occasionally necessary; the finding of a graft that is not incorporated in the surrounding soft tissue is confirmatory.
AEF represent a small subset of infected grafts. Unlike the more common, bland infected aortic grafts, patients with AEF present with some evidence of gastrointestinal bleeding. Although this can be massive, the more common scenario is a more moderate, self-limited or “sentinel” bleed. As noted above, all gastrointestinal bleeding in patients with a prosthetic aortic graft should be presumed to be secondary to an AEF until proven otherwise, and the appropriate evaluation should be initiated urgently. Notably, approximately 40% of patients with an AEF will have a second episode of bleeding within the first 24 hours after the sentinel event. The source of the bleeding or the communication with the infected graft can occur anywhere along the gastrointestinal tract, although the portion of the duodenum (i.e., junction of 3rd and 4th parts) where it crosses over the aorta/aortic graft is the most common and accounts for approximately 75% of the cases. An esophagogastroduodenoscopy (EGD) should be performed to confirm the diagnosis and/or identify other sources of bleeding. It is important to communicate the concerns
about an AEF to the endoscopist, and ideally, the surgeon should be present during the procedure. The examination should include a complete interrogation of the 3rd and 4th portions of the duodenum and may require the use of a pediatric colonoscope. Importantly, identification of a bleeding site in the stomach or proximal duodenum (e.g., gastritis, gastric ulcer) should not lead to the premature termination of the procedure. Patients with evidence of massive bleeding should likely be endoscoped in the operating room, and adherent clots should be left undisturbed to prevent recurrent bleeding. Unfortunately, a normal upper endoscopy does not exclude the diagnosis of an AEF. A contrast CT scan should be performed in conjunction with the EGD to help confirm the diagnosis. Exploratory laparotomy is occasionally necessary as a diagnostic study in this setting and requires complete mobilization of the duodenum off the aorta/aortic graft after achieving proximal and distal aortic control. Evaluation of the colon with colonoscopy is useful to complete the evaluation for gastrointestinal bleeding.
about an AEF to the endoscopist, and ideally, the surgeon should be present during the procedure. The examination should include a complete interrogation of the 3rd and 4th portions of the duodenum and may require the use of a pediatric colonoscope. Importantly, identification of a bleeding site in the stomach or proximal duodenum (e.g., gastritis, gastric ulcer) should not lead to the premature termination of the procedure. Patients with evidence of massive bleeding should likely be endoscoped in the operating room, and adherent clots should be left undisturbed to prevent recurrent bleeding. Unfortunately, a normal upper endoscopy does not exclude the diagnosis of an AEF. A contrast CT scan should be performed in conjunction with the EGD to help confirm the diagnosis. Exploratory laparotomy is occasionally necessary as a diagnostic study in this setting and requires complete mobilization of the duodenum off the aorta/aortic graft after achieving proximal and distal aortic control. Evaluation of the colon with colonoscopy is useful to complete the evaluation for gastrointestinal bleeding.
Pathogenesis
Prosthetic aortic graft infections occur in approximately 1% to 2% of all infrarenal aortic reconstructions. The incidence is approximately 0.5% to 1% in grafts isolated to the abdomen (i.e., aortoaortic, aortobiliac) and approximately 1.5% to 3% for grafts involving the groin (i.e., aortobifemoral).
The etiology of the graft infections include contamination of the surgical wound/ graft in the peri-operative period, seeding of the graft from an episode of bacteremia, erosion of the graft into the bowel or genitourinary tract, and involvement of the graft from a contiguous infectious process. Contamination of the graft in the peri-operative period is likely the most common etiology and is associated with breaks in sterile technique, concomitant foot infections, groin wound breakdowns, and emergency procedures. Notably, bacteria can be isolated from the thrombus within abdominal aortic aneurysms and aortic atherosclerotic plaques in a significant percentage of patients (15% to 40%), although the contribution of these isolates to graft infections remains unknown. AEF can result from a direct communication with the suture line, a communication with an anastomotic pseudoaneurysm, or erosion of the prosthetic graft into the bowel itself. In most cases, the graft infection precedes the AEF.
The majority (approximately 60%) of prosthetic aortic graft infections are due to Staphylococcus aureus, Staphylococcus epidermidis, and Escherichia coli, with the balance comprised of gram negative, bacteroides, and nonhemolytic streptococcus organisms. The responsible organisms vary with the time course of the infection, with Staphylococcus aureus predominating in the early postoperative period and Staphylococcus epidermidis later. Notably, organisms may not be isolated in up to 25% of the cases despite an obvious graft infection. The majority of these cases are likely due to Staphylococcus epidermidis; specific culture techniques are required to disrupt the surface biofilm and isolate this organism.
Discussion of the etiology of prosthetic graft infections merits comment about the various preventive strategies. These are comprised of standard surgical techniques and include strict sterile technique, peri-operative skin site preparation, prophylactic antibiotics with redosing during the procedure as necessary, protecting the prosthetic grafts from contact with the skin, and juxtaposition of retroperitoneal tissue between the graft and the overlying bowel.
Indications and Contraindications
Operative treatment is required for all patients with an infected aortic graft. Although appealing, long-term antibiotics have no role as the sole treatment modality. Furthermore, the mortality rate associated with untreated AEF is virtually 100%.
Pre-operative Assessment
Patients with infected aortic grafts usually have significant medical comorbidities. These comorbidities should be optimized to the greatest extent possible. Ankle-brachial indices should be obtained for all patients with additional noninvasive imaging dictated by the planned procedure. These potentially include segmental upper-extremity pressures and velocity waveforms to confirm the adequacy of the axillary artery as an arterial inflow and vein surveys of the saphenous and superficial femoral-popliteal veins. A standard aortogram and bilateral lower-extremity arteriograms should be obtained to plan the reconstructive procedure. It is rarely feasible to simply remove the graft without revascularizing the lower extremities. Indeed, remedial revascularization is complicated by the fact that the infected graft usually lies within the optimal anatomic position. Dedicated shots of the profunda femoris arteries should be obtained in the ipsilateral oblique projection. Additionally, an arch aortogram should be performed if an axillofemoral bypass is planned. Patients should be started on preoperative antibiotics, initially empiric, with adjusment based upon the results of culture data.
Operative Technique
General
The potential options for treating patients with infected aortic grafts include graft removal without revascularization, extra-anatomic bypass with graft removal, and graft removal with in situ replacement. Among these options, the extra-anatomic bypass can be performed as a single procedure or staged while prosthetic grafts, cryopreserved allografts, and autogenous veins can be used as the conduit for the in situ replacement. These various options should be within the armamentarium of surgeons caring for patients with infected aortic grafts and should be considered complementary, because they represent the appropriate choice for a specific patient/clinical scenario. Graft removal without revascularization is rarely an option given the severity of the underlying arterial occlusive disease. The list of factors that impact the choice of procedure is extensive and includes the feasibility of extra-anatomic bypass (status of axillary artery and femoral-infrainguinal runoff), patient’s comorbidities, life expectancy, presence of sepsis, suspected organism, presence of AEF, severity of bleeding in the presence of an AEF, and long-term success of the various procedures.
The staged extra-anatomic bypass with graft removal a few days later represents the most conservative, traditional approach for treating patients with infected aortic grafts. Simultaneous combined procedures (i.e., extra-anatomic bypass and graft removal) have largely been abandoned due to the observation that the staged approach is significantly safer. Furthermore, the concerns that the extra-anatomic graft will become infected during the time interval before aortic graft removal have not been realized, although there is a real risk that the extra-anatomic grafts will thrombose due to the presence of competing flow through the direct aortic reconstructions. The configuration of the extra-anatomic bypass is dictated by that of the infected aortic graft with axillobifemoral bypass (axillofemoral-femorofemoral) suitable for aortic grafts limited to the abdomen and bilateral axillofemoral bypass suitable for those involving the groin. Notably, the outflow for the axillofemoral bypass is the profunda femoris or the profunda-superficial femoral arteries, and the vessels are approached laterally through uninvolved tissue planes; the patency rates for axillopopliteal bypass are abysmal, and the procedure should generally be condemned.
The in situ replacement using the autogenous superficial femoral-popliteal vein or the neo-aortoiliac system (NAIS) is an excellent alternative for younger, healthier patients or those in which extra-anatomic bypass is not a suitable option (e.g., severe axillary artery occlusive disease). The long-term patency rates are excellent, as will be detailed below, although the magnitude of the procedure is significant. In situ replacement with either a prosthetic graft or an allograft is a reasonable option for patients with multiple comorbidities and/or low virulent organisms, although the likelihood of a recurrent graft infection is significant. Indeed, there appears to be an inverse relationship between the likelihood of successful graft salvage and the magnitude/virulence of the infectious process.
The treatment options for patients with AEF are essentially the same. However, the treatment options are usually dictated by the severity of the bleeding and the patient’s hemodynamic status. If the patient is hemodynamically stable and not bleeding, staged extra-anatomic bypass with graft removal is the optimal approach. In situ replacement with superficial femoral— popliteal vein is a reasonable alternative in this setting, although the overall experience in the literature is somewhat limited and concern has been expressed about the durability and potential for recurrent bleeding and aortic disruption. The primary concern for hemodynamically unstable patients with an AEF is to control the source of the bleeding. Specific treatments will be outlined below, but the options include a single procedure with repair of the fistula, correction of the bleeding source, and removal of the infected graft followed immediately by extra-anatomic bypass. An attractive alternative in this setting is to correct the bleeding source with an in situ prosthetic graft and repair the fistula. This essentially converts an unstable patient with an AEF to one with an infected graft that can be addressed at a later time in a semi-elective fashion. This approach emphasizes an important principle in treating patients with infected aortic grafts in that it is usually safer to treat patients with a series of smaller operations rather than a single, overwhelming procedure.
Staged Extra-anatomic Bypass and Graft Removal
A detailed description of the axillofemoral bypass is provided in Chapter 45, “Alternative, Open Revascularization for Aortoiliac Occlusive Disease.” Similarly, the approach to removing the infected aortic graft is similar to that for remedial aortobifemoral bypass procedures discussed extensively in Chapter 46, “Redo Aortobifemoral and Thoracobifemoral Bypass for Aortoiliac Occlusive Disease.” However, several technical points merit further comment and/or emphasis.
The patient is positioned on the operating table in the supine position with the arms abducted at 90°. This allows the surgeon and the assistant to stand on opposite sides of the arm and is particularly helpful when assisting a trainee. A bump can be placed along the axis of the spine and serves to drop the shoulders, thereby accentuating the mid-clavicular region. The operative field should be prepared and draped in the usual fashion, with the skin preparation extending from the chin to the toes.
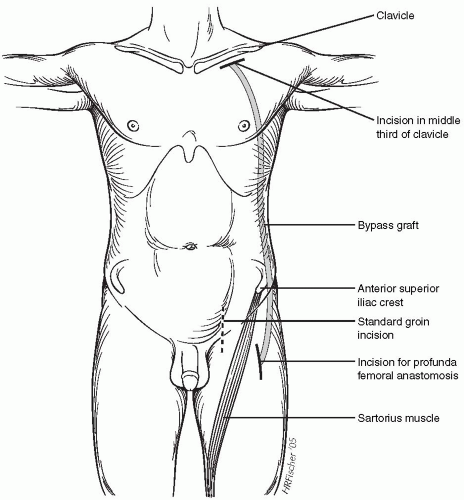
The dissection of the axillary artery is started by making an incision 1 cm below the clavicle extending along the segment that comprises its middle third (Fig. 49-2). The soft tissue and fascia overlying the pectoralis major muscle are incised along the plane of the skin incision, and the muscle fibers are separated bluntly. The axillary vein is then mobilized and retracted caudally with the assistance of a vessel loop. Several venous tributaries of the axillary vein must be transected to facilitate its mobilization. The axillary artery lies posterior and cephalad to the axillary vein and can usually be easily palpated. Approximately 3 cm of the axillary artery should be dissected free to facilitate the anastomosis, because several millimeters of the vessel are required for applying the vascular clamps. Similar to the vein, there are several small arterial branches that originate from the desired segment that can be ligated and/or clipped without sequelae. Importantly, the axillary artery should be dissected to the chest wall to facilitate placing the anastomosis as far medial as possible, thereby reducing the potential to disrupt the anastomosis with positional changes of the shoulder. It is not usually necessary to transect the pectoralis minor to expose the axillary artery. Indeed, this requirement suggests that the dissection is too far lateral on the vessel.
The location of the femoral incision is dictated by the extent of the aortic graft and whether the groins are involved. When the aortic reconstruction is confined in the abdomen (i.e., aortoaortic or aortoiliac reconstruction), a standard incision over the common femoral artery can be performed in preparation for the femorofemoral component of the axillobifemoral bypass. In the
more common scenario in which a prosthetic limb in the groin is infected, the profunda femoris artery should be exposed using a 10-cm incision along the lateral border of the sartorius muscle positioned both further distal and lateral to the standard groin incision (Fig. 49-2). The profunda femoris artery lies several centimeters deep to the skin, but it can be exposed by dissecting posterior in the thigh along the plane of the incision (Fig. 49-3A). The superficial femoral artery is frequently encountered more superficially and can be initially mistaken for the profunda. A suitable segment of the profunda femoris vessel should be dissected free, and this frequently contains several small branches that can be preserved and controlled with a vessel loop or suture. A suitable segment of the superficial femoral artery should likewise be dissected free in the event that it is patent.
more common scenario in which a prosthetic limb in the groin is infected, the profunda femoris artery should be exposed using a 10-cm incision along the lateral border of the sartorius muscle positioned both further distal and lateral to the standard groin incision (Fig. 49-2). The profunda femoris artery lies several centimeters deep to the skin, but it can be exposed by dissecting posterior in the thigh along the plane of the incision (Fig. 49-3A). The superficial femoral artery is frequently encountered more superficially and can be initially mistaken for the profunda. A suitable segment of the profunda femoris vessel should be dissected free, and this frequently contains several small branches that can be preserved and controlled with a vessel loop or suture. A suitable segment of the superficial femoral artery should likewise be dissected free in the event that it is patent.
The tunnel for the axillofemoral bypass to the profunda should be positioned posterolateral to the anterosuperior iliac crest in contradistinction to the more traditional axillofemoral bypass that courses medial to this anatomic landmark (Fig. 49-2). The tunnel can be created by advancing an 8-mm tunneler cephalad from the groin incision along the anterior axillary line and is facilitated by standing on the contralateral side of the patient. The tunneler should be advanced through the subcutaneous tissue of the lateral abdominal wall and along the anterolateral chest wall to prevent inadvertentl entry into the peritoneal cavity and/or pleural space. The tunneler is advanced along the chest wall under the pectoralis muscle and passed through the axillary incision. This can be simplified by guiding the tip of the tunneler with the fingers of the opposite hand after bluntly dissecting deep to the pectoralis muscle from the axillary incision. An 8-mm ringed PTFE graft can then be passed through the lumen of the tunneling device; it is usually not necessary to suture the graft to the inner cannula of the tunneler, because the ringed grafts have sufficient columnar strength to be advanced themselves. I prefer to tunnel the crossover femorofemoral graft below the fascia of the abdominal wall. This can be facilitated by making a vertical or diagonal incision through the inguinal ligament, then bluntly dissecting immediately below the fascia using the long finger. Occasionally, resistance is encountered in the midline that can be overcome with additional force or the use of an aortic clamp.
The axillary anastomosis can be positioned on the anterior or anteroinferior aspect of the artery, depending upon how the graft sits best (Fig. 49-4




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree