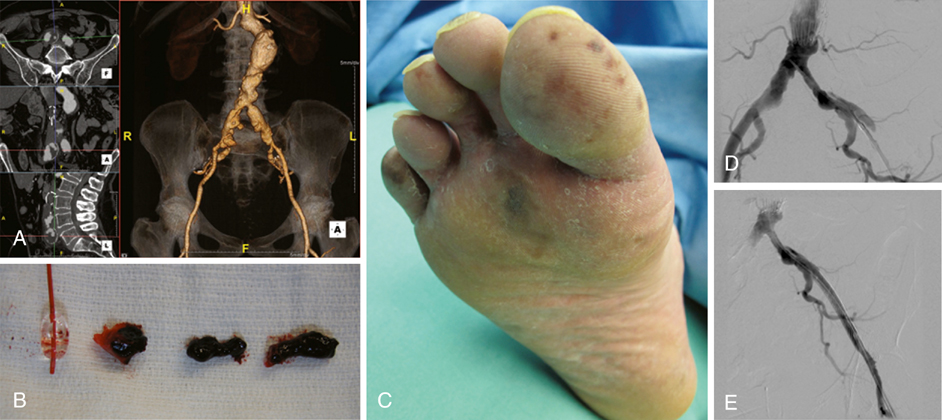
There are a number of causes of acute limb ischemia associated with aortic surgery and they can be broadly classified as embolic or thrombotic (Figure 1). Embolic subcategories include macroemboli and microemboli (see Figure 1B and C). Macroembolic sources found in diseased aortas, such as laminated or friable thrombus (see Figure 1A), can be dislodged during dissection, clamping, or endovascular manipulations and can result in obstruction of the major named arteries supplying the lower extremity. In the absence of adequate anticoagulation, thrombus can quickly develop in the obstructed artery. In cases of microembolism, the major arteries of the leg remain patent while small fragments of thrombus or debris lodge in the smaller vessels and microcirculation of the lower limb. Atheroembolism, the disruption and release of atherosclerotic plaque contents, is one type of microembolic syndrome that can produce the classic trash foot, but these microparticles can also lodge in the muscles of the legs and buttocks. Thrombotic etiologies relate primarily to inflow, conduit, and outflow problems. Poor inflow can cause thrombosis of the native artery or graft and is commonly caused by clamp injury, unappreciated proximal stenotic disease, and the use of large occlusive sheaths. Defects in the conduit, such as twisted, kinked, or compressed graft or stent graft limbs can also result in thrombotic complications. Disadvantaged outflow, such as anastomotic defects, arterial wall dissections, and flaps (see Figure 1D and E) or heavily diseased distal vasculature, can give rise to abnormal flow, leading to thrombus formation and acute limb ischemia. Lastly, systemic conditions, such as profound hypotension and hypercoagulability, can also cause thrombosis of the reconstruction or downstream arteries. After the procedure is completed and while maintaining a sterile operative field, the legs and feet can be inspected to identify most ischemic complications. The examination should include palpation of pulses and inspection of the legs and feet (see Figure 1C). The feet may be under the surgical drapes. Most importantly, knowledge of the preoperative lower extremity pulse and Doppler examinations is critical to compare with the postoperative examinations. Changes from baseline or failure to observe the anticipated improvement should be cause for concern and should prompt further investigation. Rarely, such as in cases of profunda femoris revascularization where gradual improvement is anticipated, the patient may be monitored closely without immediate intervention.
Management of Acute Limb Ischemia Complicating Aortic Reconstruction
Etiology
Diagnosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


