Chapter 68
Lymphedema
Surgical Treatment
Ruediger G.H. Baumeister
The lymphatic system is the least understood part of the vascular system. Lymphatic malformations such as chylous disorders, cystic hygromas, and lymphocysts are rare; acquired disorders such as lymphoceles and chylous effusions are also uncommon. However, local interruption and obstruction of the lymphatic vessels occur frequently from either congenital or acquired causes (see Chapter 66). In developed countries, most acquired lymphatic obstructions are iatrogenic, caused by medical procedures. In developing countries, the most frequent cause of lymphatic obstruction resulting in chronic lymphedema is filariasis.
Vessel interruption is a common pathologic process in the vascular system, and surgical repair that involves bypassing an obstruction is a well-established method in vascular surgery. These principles of surgical treatment have also been applied successfully in the lymphatic system and are the topic of this chapter.
Basis of Surgical Treatment
The development of lymphedema can be described as an imbalance between the lymphatic load—the amount of lymph that has to be cleared from a body part within a given time—and the lymphatic transport capacity—the amount of lymph that can be transported out of a body part within a given time, which is dependent on the number and function of lymphatic vessels and nodes (see Chapter 13).1 Reduced lymphatic flow due to obstruction leads to secondary tissue changes, a process that is not yet fully understood. Lymphatic outflow disorders are manifested mainly with advanced secondary changes and chronic lymphedema, a condition that can be difficult to treat unless the underlying problem of reduced lymphatic outflow is resolved. Historically, secondary tissue changes leading to excess fibrous and adipose tissue were treated solely by excisional procedures, without correcting the underlying cause. However, modern surgical concepts have been developed to attempt to correct the underlying pathophysiologic mechanism to the extent possible.
Enhancing the number of functional lymphatic vessels through surgical reconstruction is the most natural way to address the problem and is especially beneficial when the reconstruction is performed before secondary tissue changes occur. Thus, it is not surprising that the surgical reconstruction of lymphatic vessels in selected patients has yielded excellent results. One obstacle is the small dimension of lymphatic vessels, which can be overcome only with advanced microsurgical approaches. Once secondary tissue changes have evolved, additional efforts may be required. One of the less invasive methods in current use is liposuction.
Because conservative therapy consisting of limb elevation, compression garments, complex decongestive therapy, and compression pump therapy should be the first step (see Chapter 67), the question arises whether and at what time surgery is indicated. If the only purpose of surgery is resection, it is wise to reserve it as a last option. If, however, surgical reconstruction is possible, this procedure should be considered and offered to the patient early in the course of lymphedema.
Historical Perspective
Excisional Operations
Surgical Excision
The most radical excisional approach is the classic operation first described by Charles in 1912.2 It involves complete and circumferential resection of the skin, subcutaneous tissue, and deep fascia, followed by split-skin grafting. However, this procedure is associated with significant complications, and follow-up studies revealed hyperkeratosis, papillomatosis, and ulcerations in the grafted areas.3,4 Modifications of this technique, using the resected skin for grafting and performing the surgery in two stages,5–11 reduced the surgical trauma and the rate of complications.
The techniques of resecting the fascia and subcutaneous tissue and creating skin flaps were based on the proposals of Auchincloss, Fontaine, Homans, and Servelle.12–15 In contrast to the Charles procedure, the flap procedure yields a better cosmetic result, but it requires sufficient healthy skin in the affected extremities. Limited resection of fascia, subcutaneous tissue, and skin was suggested by Sistrunk.16,17
Liposuction
A less invasive way to reduce the amount of subcutaneous tissue is liposuction. It was first described by Illouz as a method for treating lymphedema.18 More recently, Brorson and Svensson demonstrated lasting volume reduction if elastic compression garments are worn after the surgical procedure; this can result in an extremity that is even slimmer than the healthy limb.19
Functional Procedures
Early Techniques
The first attempts to divert lymph from the subcutaneous to the muscular compartment through partial or complete resection of the fascia were described by Lanz and Kondoleon.20–22 Redirection of lymph from the superficial to the deep compartment is also a component of the Thompson method. Thompson resected the fascia along with parts of the subcutaneous tissue, created a flap in two stages, and de-epithelialized the rim of the flap to allow the outflow of lymph. Subsequently, he buried the flap near the deep vessels to facilitate the creation of spontaneous lymphatic anastomoses.23–30
Facilitating spontaneous lympholymphatic anastomoses within the subcutaneous tissue has been the aim of several methods, and a variety of flaps have been created for transposition into the edematous area.31–35 Goldsmith and coworkers proposed the creation of a pedicle from the greater omentum,36,37 and Kinmonth and associates proposed the construction of an enteromesenteric bridge using a pedicle of ileum (denuded of its mucosa) and its mesentery (rich in lymphatics) to drain lymph out of the edematous leg tissue.38
The use of veins for the reconstruction of an interrupted lymphatic system was investigated by Holle and Mandl experimentally and performed in two patients clinically.39,40 Campisi and colleagues reported on a larger series using this technique.41
Modern Techniques
Currently, the most common way to drain lymph from edematous tissue is the construction of connections between the lymphatic (Fig. 68-1) and venous systems in the periphery. The first reports on lymphonodular and lymphovenous anastomoses were provided by Laine and Howard,42 Nielubowicz and Olszewski,43 Rivero and coworkers,44 and Allen and Taylor.45 Degni designed a special needle to facilitate the insertion of lymphatic vessels into veins.46,47 Further improvements were described by O’Brien and colleagues using microsurgical techniques.48,49 In some patients, excisional methods were combined with lymphovenous shunting. A large cohort of patients successfully treated with microsurgical lymphovenous anastomosis was reported by Campisi and associates.50
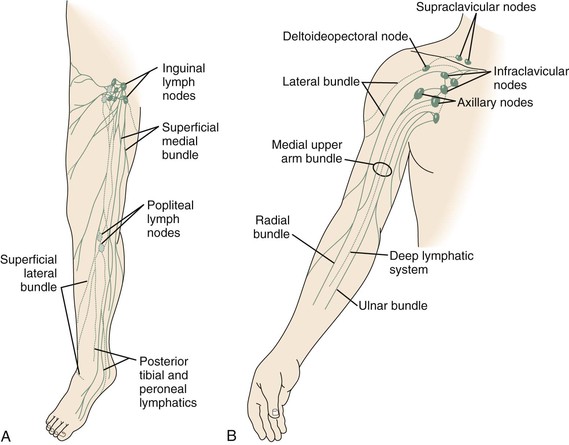
Figure 68-1 A, Superficial lymphatic system of the lower extremity. B, Lymphatic system of the upper extremity. (Courtesy Mayo Foundation.)
However, experimental studies revealed problems with thrombotic occlusion at the site of anastomosis, with a patency of 20% after 5 months of follow-up.51–53 Specific preparations that ensured an undisturbed connection to the venous valve led to an improved patency rate of 44% after 6 months.54,55 Gloviczki and colleagues reported on results of experimental microsurgical end-to-end anastomoses between normal femoral lymph vessels and a tributary of the femoral vein in dogs and noted a 50% patency rate up to 8 months after the operation.56
Direct reconstruction of the lymphatic system became a possibility only after the development of microsurgery. Before that time, it was commonly thought to be impossible to anastomose lymphatic vessels because of their extremely small diameters. Hence, Danese approximated lymphatic vessels as close to each other as possible and waited for spontaneous regeneration. He was able to demonstrate transport of contrast medium (through the lymphatics) with this technique.57 In a patient with lymphedema of the arm, he mobilized two lymphatic channels proximally and distally, approximated them in the axilla, and achieved a reduction in edema.58 Subsequent approaches included interpositioning veins between lymphatic vessels,39–41 implanting microsurgical lymph node grafts,59,60 and implanting free flaps with lymphatic vessels.61,62
The question of optimal reconstruction material has been the subject of two experimental studies. In a series of 14 rats, 100% of the autologous lymphatic grafts were patent (observations made between days 7 and 119 postoperatively), whereas allogeneic lymphatic grafts were patent only until day 21 after transplantation. When lymphatics were replaced by small veins (n = 10), a patency of 70% was observed. Expanded polytetrafluoroethylene implants (n = 10) used as lymphovascular conduits were already thrombosed by day 7.63 In a comparison of lymphatic and venous interpositional autografts in 71 dogs, all 26 lymphatic autografts remained patent up to the end of the observation period at 24 weeks. Of 30 venous interpositional autografts, only 4 were patent after 1 week. None of the lympholymphatic anastomoses with silicone tubing showed patency at any time.64
Acland and Smith were the first to attempt to anastomose lymphatic vessels.65–67 The first successful therapeutic lympholymphatic graft was performed in 1980 by Baumeister in a patient with unilateral lymphedema of the lower extremity.68–71 This followed extensive animal experiments on thoracic duct transplants in rats72 and the treatment of experimental lymphedema in dogs using lymphatic autografts.67
Preoperative Planning
Visualizing Lymphatic Vessels
For vascular surgeons, direct visualization of a single lymphatic vessel would be the ideal technique for preoperative evaluation and planning.
Lymphography
Direct contrast lymphography, using oily contrast medium and invasive administration through dissected lymphatic vessels, was introduced by Kinmonth and greatly advanced our knowledge of the lymphatic system.73 However, owing to the invasiveness of the procedure (and injury to the lymphatic vessels and lymph nodes), it was found to worsen lymphedema and is rarely used today. Indirect contrast lymphography, using a water-soluble contrast medium injected subepidermally, cannot visualize lymphatic vessels as well as direct lymphography and gained only limited use.74 In primary lymphedema, this technique might be used to evaluate whether there are any lymphatic vessels present in the periphery and, if so, whether they might be able to transport lymph toward a proximally performed anastomosis.
Magnetic Resonance Imaging
Attempts to visualize lymphatic vessels with magnetic resonance imaging (MRI) and subdermally administered contrast medium have been promising. This technique may be useful in the future for preprocedure planning and postoperative assessment of the patency of lymphatic reconstructions.75 For the detection of vascular lymphatic malformations, MRI is extremely valuable both with and without contrast medium.
Lymphoscintigraphy
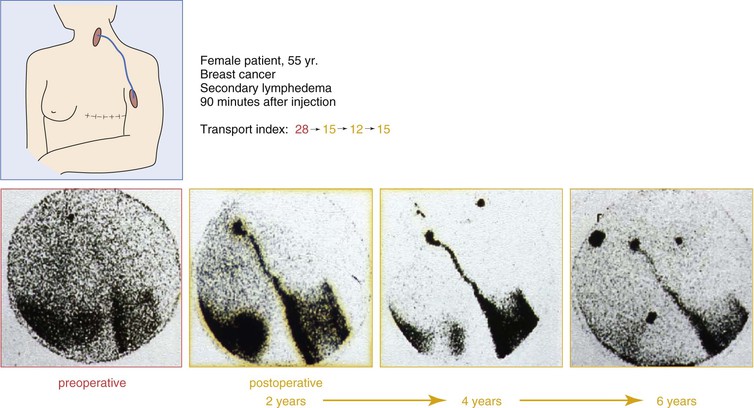
The most important test to evaluate chronic lymphedema and to plan surgical treatment is lymphoscintigraphy. It can be repeated and used for treatment planning as well as for follow-up. It not only evaluates function but also visualizes routes of lymphatic transport. The lymphatic transport index summarizes the findings derived from lymphoscintigraphic studies and allows a semiquantitative evaluation of lymphatic flow without the need for standardized physical movements by the patient. The transport index ranges from 0 for an optimal lymphatic outflow to 45 for no visible transport; normal values are less than 10. It also provides a good basis for follow-up studies and can show lymphatic transport along the route of lymphatic grafts.76,77 In measuring lymph transport at regions of interest, it is critical to standardize the dose of radiopharmaceutical and the physical activity of the patient during the procedure.78
Dye Injection
Another diagnostic tool that can be used to identify lymphatic channels is the subepidermal injection of a vital dye (patent blue dye in Europe; isosulfan blue [Lymphazurin] dye in the United States). Normally, lymphatic transport is visualized in the superficial lymphatic collecting system. In pathologic situations, dermal backflow leads to the pooling of contrast medium within the skin, resulting in a cloudlike appearance. Because allergic reactions have been reported, staining of lymphatic vessels with patent blue or isosulfan blue dye is generally performed during surgery under general anesthesia.
Lymphatic Donor Site Assessment
For lymphatic grafting, it is critical to choose and carefully evaluate the proper harvest site for lymphatic vessels to avoid the development of edema secondary to the procedure. Thus, the donor lower extremity must be evaluated by lymphoscintigraphy before harvesting. During the harvest, the narrowing lymphatic system at the medial aspect of the knee and the groin must be left untouched, and all stained lymphatic vessels other than those used as grafts should be left in place. A study including 80 patients with arm edema showed that when this method was used, the harvest site and the untouched leg were not different in size.71
Patient Risk Assessment
Because this type of surgery is performed in the subcutaneous tissue, the surgical risk is generally low, and the procedure is well tolerated. Peripheral lymphovenous shunting is often performed under local anesthesia and is unproblematic as long as the patient tolerates local anesthetics. Because the application of patent blue or isosulfan blue dye can lead to allergic reactions, it should be used only under general anesthesia. Excisional methods typically involve more surgical trauma and the possibility of greater blood loss. Therefore, it is sometimes advisable to perform large excisional operations in two stages. For surgical intervention within the abdomen and thorax, the usual preoperative risk assessment must be done (see Chapter 31).
Surgery
Autologous Lymphatic Grafting
Indications
Lymphatic vessel grafts can be attempted for the treatment of secondary lymphedema caused by localized obstruction or interruption of lymph vessels and lymph nodes, such as the lymphedema of the arm that develops after breast cancer surgery because of the excision of axillary lymph nodes and possible radiation treatment (see Fig. 68-1B). Patients with unilateral lower limb lymphedema due to the excision of inguinal or pelvic lymph nodes or pelvic irradiation for malignant disease are also potential candidates for such procedures. Transplantation of lymphatic vessels can be attempted in patients with primary lymphedema if it is caused by localized lymphatic obstruction or atresia, such as unilateral atresia of the pelvic or inguinal lymphatic system.
Surgical intervention should be considered only after a trial of conservative therapy. Conservative treatment should be continued for at least 6 months because spontaneous regression has been reported. However, if conservative therapy is unsuccessful during this time frame, reconstruction should be attempted soon to avoid secondary tissue changes. Unfortunately, treatment is often delayed. In my experience, the mean time between the onset of edema and the patient’s presentation for surgery is more than 7 years.
Preoperative Evaluation
Bilateral isotope lymphoscintigraphy is performed in every patient. Both lower extremities are tested to determine a safe harvest site of lymph vessels for the transplant. Only limbs with normal lymphatic transport capacity are used as donors of lymphatic grafts.
In primary lymphedema, indirect lymphography or MRI lymphography is performed in an attempt to visualize lymphatic vessels distal to the occlusion that are suitable for anastomosis. In all cases of lymphedema, malignant disease must be excluded before surgery.
Surgical Technique
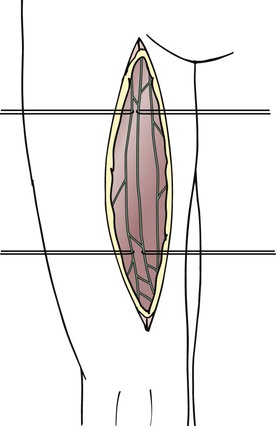
The lymphatic grafts are harvested from the patient’s thigh (Fig. 68-2). The ventromedial lymphatic bundle contains up to 16 lymphatic vessels.79 About one to four lymphatic collectors are dissected in the medial area of the thigh, and great care is taken to spare the lymphatic system where it narrows at the level of the knee and groin. Additional peripheral branches often exist, and these can be dissected as well to create a greater number of peripheral anastomoses.
For free transfer of the graft, a ligature is placed on the lymphatic vessel selected as a graft beneath the inguinal lymph nodes with 6-0 polyglactin 910. One thread is left long to facilitate handling of the graft thereafter. Proximal to the ligature, the lymphatic vessel is transected. At the distal end of the proposed graft, the lymphatic vessel is transected proximal to the level of the knee. The lymphatic vessel beneath the transection site is occluded either by placing a suture or by using coagulation to avoid lymph leakage.
If a transposition procedure is performed, the grafts are transected distally after double ligation and tunneled subcutaneously superior to the pubic symphysis to the contralateral side, where end-to-end lympholymphatic anastomoses are performed. In free transfers as well as in transposition procedures, the graft has to be pulled from one incision to the other (e.g., from the upper arm to the neck or between inguinal regions). To avoid any friction during tunneling, tubes (suction catheters) are placed between the two incisions according to the proposed route of the grafts. Thereafter, the grafts themselves can be pulled through the tubes without tension. After removal of the tubes, the grafts remain undisturbed in place within the subcutaneous tissue.
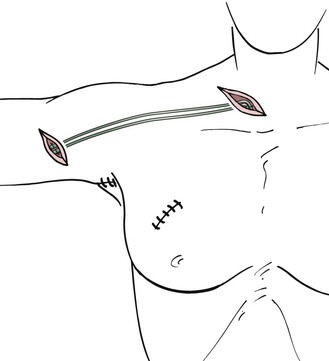
Arm and Neck.
For arm edema as a result of interventions in the axilla, the grafts are interposed between ascending lymphatic vessels in the upper arm and lymphatic vessels or lymph nodes in the neck (Fig. 68-3). In the upper arm, lymphatic vessels are usually epifascial (if not, they may be located subfascially in proximity to the vessels) and are best sought from an oblique incision made medially and superior to the route of the brachial vessels. The search is performed under the microscope with a medium (3× to 10×) magnification. In the early stages of lymphedema, the lymphatic vessels have a gray, shiny appearance, and the lumen can be seen clearly after transection. As the lymphatic vessels undergo fibrosis in later stages of lymphedema, it becomes more difficult to discriminate between small nerves and fibrous cords. In this case, the final decision about the potential use for grafting can be made only after transection of the structure.
The walls of lymphatic vessels are thinner in the neck than in the arms and legs. Injection of a vital dye in the hair-bearing parietal area above the ear facilitates the search for appropriate vessels. If the lymphatic vessels stain appropriately, recognition is easy. However, suturing in this area is often difficult because of the collapsing, thin-walled vessels. If this is the case, it is also possible to suture the grafts to lymph nodes. A superficial incision is made in the capsule of the node, and the graft is connected with approximately three single interrupted sutures.
To position the grafts between the sites of anastomosis, tubing from a drain is placed in the subcutaneous tissue between the incisions in the upper arm and neck. Subsequently, the grafts are pulled through the wet drain gently and without friction. After removal of the tube, the grafts remain in the subcutaneous tissue free of tension.
Leg.
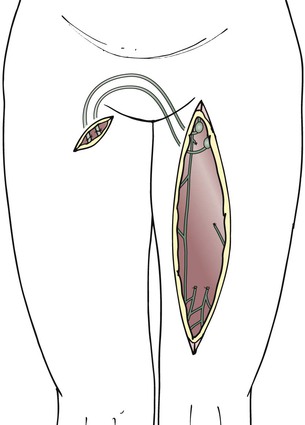
For unilateral edema of the lower extremity, the grafts remain attached to the inguinal lymph nodes, and the distal ends of the grafts are transposed to the opposite thigh with the help of tubing from a drain, which is temporarily interposed between the two incisions at the thigh (Figs. 68-4 to 68-6).
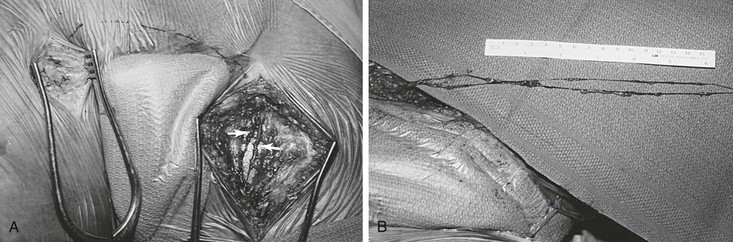
Figure 68-5 A, Exposure of lymph vessels for suprapubic transposition. Note that two major lymph vessels of the left thigh will be used for grafting (arrows). B, Two lymphatic grafts divided at the distal thigh are prepared for grafting. (Courtesy Mayo Foundation.)
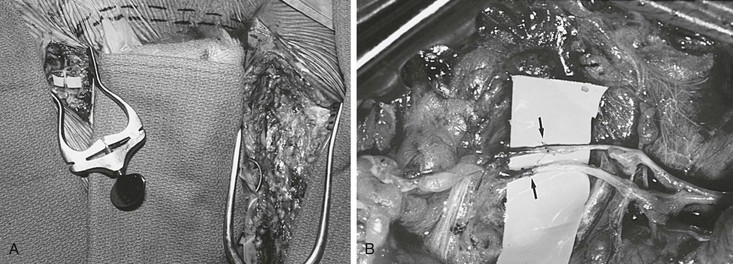
Figure 68-6 A, Completed suprapubic lymph graft with two lympholymphatic anastomoses in the right groin. Dashed line indicates the position of the suprapubic lymphatic grafts. B, Magnified photograph of two end-to-end lympholymphatic anastomoses (arrows) performed with 11-0 interrupted monofilament sutures. (Courtesy Mayo Foundation.)
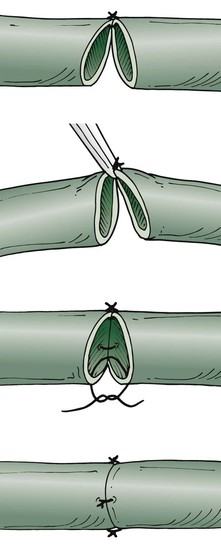
The tension-free technique is used to anastomose the lymphatic vessels under the operating microscope with maximal magnification (Fig. 68-7). The suture opposite the surgeon should be performed first. Because of the fragility of the vessels, the vessels are not turned over. Only the back wall is lifted as far as necessary to place a dorsal stitch. One or two additional single stitches complete the anastomosis. In my experimental studies of suture material, absorbable polyglactin was superior to nonabsorbable material. Currently, 10-0 absorbable suture (polyglactin 910) is the thinnest material available, used on a BV-75-4 needle.
Postoperative Treatment
After surgery, elastic bandages are applied, and an elastic compression garment is prescribed for 6 months, at which time discontinuation of the garment is considered. Antibiotics and erysipelas prophylaxis are given for 6 months because of the reduced immunologic resistance in patients with lymphedema. Low-molecular-weight dextran or 6% hydroxyethyl starch infusions are given for 1 week postoperatively to improve lymph flow.
Results
Results were evaluated by volume estimation based on circumferential measurements along the limb in increments of 4 cm. Further, lymphatic outflow was measured semiquantitatively by the lymphatic transport index based on lymphoscintigraphic studies.76 Direct visualization of the grafts was difficult because, with lymphangiography using water-soluble contrast medium, the lymphatic vessels can generally be visualized only over short distances. However, in several patients, patent grafts could be demonstrated more than 10 years after grafting with this technique.80
In a series of 127 patients with arm edema, a significant volume reduction was achieved, from a mean of 3368 cm3 preoperatively to a mean of 2567cm3 after 8 to 10 days (P < .001). At a mean follow-up of 2.6 years, the mean volume was 2625 cm3 (P < .001). In a group of 8 patients with long-term follow-up of more than 10 years, the mean volume was reduced to 2273 cm3 from a mean preoperative volume of 3004 cm3 (P < .001).
In 81 adult patients with unilateral edema of the lower extremities, the mean preoperative volume of 13,098 cm3 was reduced to a mean of 10,578 cm3 at the time of hospital discharge (P < .001). After a mean follow-up period of 1.7 years, the volume reduction was sustained, with a mean volume of 11,074 cm3 (P < .001). In a group of 12 patients observed for more than 4 years, the volume was reduced to 10,692 cm3 (P < .001).71 The following complications were observed: two cases of erysipelas in the initial group of patients, one lymphocyst at the harvesting site, and swelling of the lower leg after thrombosis in one patient.
Lymphoscintigraphic studies were performed during a follow-up period of 8 years in 20 patients (12 upper extremities, 8 lower extremities). Of the 20 patients, 17 showed improved lymphatic outflow. In five patients, patent grafts could be demonstrated directly by visualizing the routes of activity.81 Figures 68-8 and 68-9 show lymphoscintigraphic studies along with the transport index for two patients with a follow-up period of 6 years. A quality of life study in 212 patients who had undergone lymphatic grafting showed a significant improvement in the physiologic and the psychologic conditions as well.82
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree