Lung Transplantation
GENERAL PRINCIPLES
• This chapter briefly touches on the background of lung transplantation, common terminology, and candidate selection. The primary focus, however, is on postoperative management of adult lung transplant patients, including a review of immunosuppressive agents as well as common complications and their management.
• There are three general arms to the organ transplantation system in the United States:
 United network for organ sharing (UNOS) operates the organ procurement and transplantation network (OPTN) and maintains a national registry for organ matching.
United network for organ sharing (UNOS) operates the organ procurement and transplantation network (OPTN) and maintains a national registry for organ matching.
 Organ procurement organizations (OPOs) are nongovernmental organizations that recover organs in their respective service areas and allocate them based on UNOS policies.
Organ procurement organizations (OPOs) are nongovernmental organizations that recover organs in their respective service areas and allocate them based on UNOS policies.
 Transplant centers: as of November 2011, there were 246 transplant centers in the United States and 63 of these were performing lung transplantation.
Transplant centers: as of November 2011, there were 246 transplant centers in the United States and 63 of these were performing lung transplantation.
• The most common underlying lung diseases leading to transplantation are chronic obstructive pulmonary disease (COPD)/emphysema (including α1-antitrypsin deficiency), idiopathic pulmonary fibrosis (IPF), cystic fibrosis (CF), sarcoidosis, and idiopathic pulmonary arterial hypertension (IPAH).
• Heart–lung transplantation is generally reserved for patients with Eisenmenger syndrome and an uncorrectable congenital heart defect.
• The majority of lung transplant recipients are between 18 to 64 years of age, though the percentage of recipients >65 years has increased in recent years.1
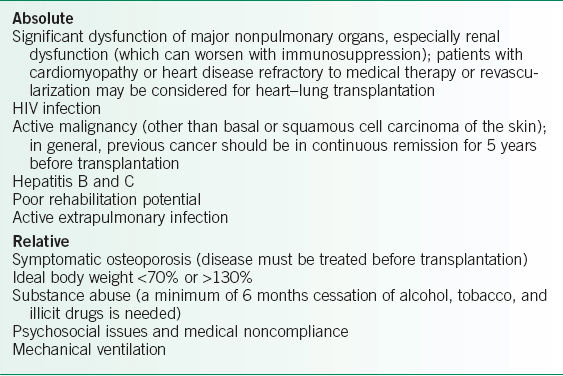
• Candidates should be in relatively good health except for their lung disease. When referring a patient for transplantation, absolute and relative contraindications must be considered (Table 29-1).2–4
DIAGNOSIS AND CANDIDATE EVALUATION
Donor Selection
• Donor organs remain in short supply.
• Given the limitation in the organ pool, donor criteria have become increasingly liberalized. Standard criteria for acceptance are listed in Table 29-2.5
• Efforts to broaden the donor pool include acceptance of marginal donors, donation after cardiac death (so-called DCD donor), and development of ex vivo organ reconditioning protocols.
• Potential donors are screened for social and medical history, physical examination findings, cause of death, vital signs, documentation of arrest or hypotensive episodes, use of vasopressors and/or hydration, echocardiogram and ECG, and bronchoscopy.
• Donors are also tested for HIV, hepatitis B and C, human T-cell leukemia virus type 1 (HTLV-1), syphilis, and cytomegalovirus (CMV, pretransfusion preferred). Organs that are positive for HIV or HTLV-1 are excluded from transplantation.
• Malignancy usually prevents transplantation, except for localized skin cancers, cervical cancer, or neurologic tumors that rarely metastasize.
TABLE 29-1 CONTRAINDICATIONS TO LUNG TRANSPLANTATION
Recipient Selection and Organ Allocation
• Each transplant center has its own specific evaluation requirements. Patients usually undergo a thorough battery of history, physical examination, pulmonary function and imaging tests. Cardiac, kidney, liver, and other vital organ functions are evaluated as needed based on the results of screening tests.
• Following this evaluation, the suitability for transplantation and appropriate timing for listing are decided.
• The donor and the recipient are matched for ABO blood groups, height, and the absence of circulating antidonor HLA antibodies (discussed further in the section on Rejection).
• Prior to 2005, priority for lung organ allocation was determined primarily by waiting time. In 2005, the Lung Allocation System (LAS) was developed with the goal to allocate organs based primarily on medical urgency and expected outcome (i.e., success) after transplantation.6
• This new system generally favors high-urgency candidates and transplantation in sicker patients, although the overall outcomes after transplantation have not been affected substantially based on current data.
TABLE 29-2 STANDARD LUNG TRANSPLANT DONOR CRITERIA

Surgical Considerations
• Single (SLT) and bilateral lung transplantation (BLT) are possible for COPD, α1-antitrypsin deficiency emphysema, IPF, IPAH, and in some cases of Eisenmenger syndrome.
• BLT is mandatory for diffuse bronchiectasis associated with CF or other diseases.
• Heart–lung transplantation is usually reserved for complex congenital heart diseases with pulmonary hypertension.
• BLT is the most common procedure performed currently.
TREATMENT
Immunosuppressive Therapy
• Induction: Some, but not all centers use induction immunosuppressive therapy immediately following transplantation. Treatment options have included interleukin (IL-2) receptor antagonists, antilymphocyte antibody preparations, or alemtuzumab (anti-CD52).7
• Maintenance: Immunosuppression strategies vary among transplant centers but most use a triple-drug maintenance regimen consisting of a corticosteroid (methylprednisolone perioperatively, followed by prednisone), an antimetabolite (azathioprine or mycophenolate mofetil [MMF]), and a calcineurin inhibitor (cyclosporine [CsA] or tacrolimus).7
Specific Agents
Corticosteroids
• Steroids have anti-inflammatory effects in both the innate and adaptive arms of the immune system. Dosing is variable.
• Metabolism and excretion: Hepatic metabolism, including cytochrome P450-3A4 isoform (CYP3A4), and urinary excretion.
• Interactions: Barbiturates, phenytoin, rifampin, and St. John’s wort decrease corticosteroid effectiveness by inducing CYP3A4. Conversely, inhibitors of CYP3A4, such as azole antifungals and macrolides, may increase steroid levels. Steroids may also increase CsA levels and potentiate aspirin- or NSAID-induced gastritis.
• Adverse drug reactions: Complications are common with chronic steroid use and include skin thinning, impaired wound healing, fat redistribution, hypertension, hypokalemia, hyperglycemia, adrenal insufficiency, osteoporosis, and mental status changes (ranging from restlessness and poor sleep to agitation and steroid psychosis). Corticosteroids may also increase or decrease the prothrombotic effect of warfarin.
Azathioprine
• Azathioprine is a purine analog that inhibits DNA and RNA synthesis, ultimately blocking proliferation of activated lymphocytes.
• Initial dosing is 1–3 mg/kg PO/IV daily.
• Bioavailability: Azathioprine is well absorbed after oral administration. Azathioprine and its metabolite 6-mercaptopurine are 30% bound to plasma proteins.
• Metabolism and excretion: Hepatic metabolism and urinary excretion.
• Interactions: Allopurinol may reduce metabolism and increase levels of azathioprine. Drugs with bone marrow suppression or toxicity should be avoided, as the effects can be additive. Warfarin levels may increase via unknown mechanisms.
• Adverse drug reactions: Bone marrow toxicity can occur (thrombocytopenia, anemia, and leukopenia). Leukopenia is especially common in patients with mutations in thiopurine S-methyltransferase, which can be screened with genetic testing if needed. Gastrointestinal (GI) side effects can include hepatitis, cholestatic jaundice, and pancreatitis.
Mycophenolate Mofetil
• MMF was initially developed as an antibiotic/antineoplastic/antipsoriatic agent. It is a selective, noncompetitive, and reversible inhibitor of inosine monophosphate dehydrogenase, blocking de novo purine synthesis. As B and T cells lack the salvage pathway of purine synthesis, they are selectively inhibited.
• Initial dosing is 1–1.5 g PO/IV bid.
• Bioavailability: MMF is given as an ester derivative owing to poor absorption. In this form it is rapidly absorbed orally. It is 97% albumin bound in plasma.
• Metabolism and excretion: MMF is rapidly hydrolyzed to an active metabolite mycophenolic acid (MPA) in the liver. Also, it is later inactivated in the liver by glucuronidation. MPA is eliminated primarily in the urine as MPA glucuronide. In renal failure, accumulated MPA glucuronide may be converted to MPA, causing toxicity.
• Interactions: Relatively few drug interactions occur. Antacids may reduce absorption. Cholestyramine and antibiotics that alter gut flora can decrease levels by reducing enterohepatic circulation. Drugs that interfere (e.g., probenecid) or compete for renal tubular secretion may increase MPA glucuronide levels. High doses of salicylates may increase free MPA levels.
• Adverse drug reactions: MMF is generally well tolerated with GI side effects being most common (abdominal pain, nausea, vomiting, dyspepsia, diarrhea); these can be overcome by splitting doses or administering the drug with small amounts of food. Bone marrow toxicity is seen as well (anemia, leukopenia, and thrombocytopenia).
• Monitoring: Therapeutic monitoring is not routinely performed. Concentrations may be monitored in renal failure or coadministration with CsA.
Cyclosporine
• CsA is a fat-soluble fungal polypeptide that inhibits production of IL-2 from CD4+ cells. It binds cyclophilin in lymphocytes, and the complex then binds calcineurin, inhibiting cytokine gene transcription and lymphocyte proliferation.
• Initial dosing is 5–10 mg/kg/d split into two doses.
• Bioavailability: Oral bioavailability is variable and dependent on the drug formulation (sandimmune 10–90%, neoral 30–45%). It is also bile dependent and can be influenced by fat intake, diarrhea, and GI motility. CsA is mostly distributed outside of the blood volume and the fraction in plasma is 90% lipoprotein bound.
• Metabolism and excretion: CsA is extensively metabolized in liver and intestine (CYP3A4). Elimination is primarily by excretion of metabolites in the bile. Only a small fraction is excreted unchanged via GI and genitourinary tracts.
• Interactions: Drug interactions are very common as a result of CYP3A4 induction or inhibition. Drugs that decrease CsA levels include rifampin, phenytoin, carbamazepine, phenytoin, St. John’s wort, and hydroxymethylglutaryl (HMG) coenzyme A reductase inhibitors. Increased levels are seen with azole antifungals, macrolides, calcium channel blockers (verapamil and diltiazem; nifedipine has less effect), and grapefruit juice. Many nephrotoxic drugs have synergistic toxicity with CsA. Potassium-sparing diuretics should be avoided owing to the potential for hyperkalemia. Concomitant use of HMG coenzyme A reductase inhibitor therapy increases the risk of myopathy and rhabdomyolysis.
• Adverse drug reactions: Renal side effects are common (hyperkalemia, hypomagnesemia, hypertension). Metabolic side effects include hyperlipidemia, gout, osteoporosis, hirsutism, and hyperglycemia. Neurologic effects include tremors, peripheral neuropathy, headaches, mental status changes, and, in rare instances, reversible posterior leukoencephalopathy. Gingival hypertrophy (especially in conjunction with nifedipine), a thrombotic thrombocytopenic purpura–like syndrome, and hepatotoxicity can be seen as well.
• Monitoring: Therapeutic monitoring is performed due to intra- and interpatient variability of absorption, metabolism, and excretion, as well as the considerable side effect profile. Levels measured include trough, area under the curve, and C2 pseudopeak levels. Target levels vary with time interval after transplant, organ type, and rejection history.
Tacrolimus
• Tacrolimus is a fungal-derived macrolide that inhibits IL-2 production. It binds to immunophilin FKBP12, and blocks calcineurin activity in a fashion similar to that of CsA.
• Initial dosing range is ∼0.1 mg/kg/d PO divided into two doses.
• Bioavailability: Oral bioavailability is poor (20–25%) but not bile acid dependent. It is fat soluble, and ∼80% of serum drug is RBC membrane bound.
• Metabolism and excretion: Tacrolimus is metabolized in the liver and intestine (CYP3A4). Tacrolimus is excreted unchanged in bile, thus there is no need for adjustment in renal failure or hepatic disease.
• Interactions: Similar to those with CsA.
• Adverse drug reactions: Similar to those with CsA.
• Monitoring: Trough levels are routinely used (and correlate with area under the curve measurements).
Sirolimus
• Sirolimus is a fungal-derived macrolide, also known as rapamycin. Unlike the calcineurin inhibitors tacrolimus and CsA, the sirolimus–immunophilin complex inhibits the mammalian target of rapamycin (mTOR) and blocks cytokine-mediated cell cycling and B- and T-cell function.
• Initial dosing is 2 mg/d. It is diluted with water or juice (except grapefruit juice). A long half-life allows for once-daily dosing.
• Bioavailability: Sirolimus is rapidly absorbed after oral administration but has poor bioavailability (∼14% with the oral solution but higher with tablets). It is 92% bound to plasma proteins.
• Metabolism and excretion: It is metabolized in the liver and intestine (CYP3A4). More than 90% is eliminated via the gut.
• Interactions: Similar to those with CsA. There is marked interaction with CsA itself, increasing the levels of CsA by >300%. CsA can be dosed 4 hours before sirolimus (but this complicates monitoring of blood levels).
• Adverse drug reactions: Side effects include hypertension, hypercholesterolemia, and hypertriglyceridemia. Bone marrow toxicity (thrombocytopenia and anemia) may occur. Other effects include interstitial pneumonitis and hepatotoxicity. Sirolimus has a boxed warning regarding immediate use after lung transplant, as it has been associated with bronchial anastomotic dehiscence. It can be safely used later (after anastomotic healing), but caution is warranted if other surgeries are required.
• Monitoring: Monitoring is essential as target levels also depend on whether CsA or tacrolimus is used.
Interleukin-2 Receptor Antagonists
• IL-2 receptor antagonists are chimeric murine–human monoclonal antibodies. They bind the IL-2 receptor on the surface of activated T lymphocytes and inhibit proliferation and differentiation of T cells. Basiliximab is a true chimeric antibody (25% mouse) used for induction immunosuppression. Daclizumab is a humanized antibody (10% mouse) that is no longer available.
• Basiliximab has a half-life of ∼14 days. It is given as a 20-mg IV infusion once before transplant and then again on the fourth day posttransplantation.
• Adverse effects: Basiliximab is fairly well tolerated, much better than predecessors OKT3 and muromonab-CD3. Side effects are generally similar to placebo but there remains a theoretical risk for infection and posttransplant lymphoproliferative disorder (PTLD). A severe, acute hypersensitivity syndrome (including a pulmonary edema/acute respiratory distress [ARDS]-like picture) can occur with basiliximab and is a contraindication to continued use.
Antithymocyte Globulin
• Antithymocyte globulin (ATG) is a polyclonal antilymphocyte globulin used for treatment of rejection and also for induction immunosuppression in some centers. Atgam is derived from horses, whereas thymoglobulin is of rabbit origin. There is profound B- and T-cell depletion after administration owing to complement-mediated cytolysis of antibody-coated cells.
• Dosing: Atgam: 10–20 mg/kg IV infusion. Thymoglobulin: 1–1.5 mg/kg IV infusion. Atgam has a half-life of 6 days, whereas thymoglobulin has a half-life of 30 days. Thymoglobulin is ∼10 times more potent than atgam.
• Adverse drug reactions: There are numerous reactions, including flu-like symptoms secondary to cytokine release syndrome (IL-1, IL-6, tumor necrosis factor-α). These symptoms can be attenuated with premedication (using a combination of prednisone, acetaminophen, diphenhydramine, and IV fluids). There is a potential risk of infection and PTLD, but the data in lung transplantation are variable. Leukopenia is the most serious complication of therapy. Thrombocytopenia may complicate therapy and anaphylaxis is documented but rare.
• Monitoring: Some centers monitor CD3+ levels to gauge adequacy of therapy.
Other Agents
• Alemtuzumab (anti-CD52) has been used by a few centers for induction immunosuppression or for treatment of rejection. However, this drug is no longer widely available and is used under a special distribution program.
• Azithromycin is a macrolide antibiotic that has demonstrated efficacy to delay the development of bronchiolitis obliterans syndrome (BOS) and chronic rejection in several studies. Dosing schedules are usually three times per week.
• Leflunomide is an antimetabolite that blocks pyrimidine synthesis and lymphocyte proliferation, similar to purine synthesis inhibitors.
• Rituximab (anti-CD20) is a chimeric monoclonal antibody that destroys B cells and commonly used for connective tissue diseases, such as lupus. It is also used in the treatment of antibody-mediated rejection (AMR) in some centers.
• Bortezomib is a proteasome inhibitor used in the treatment of multiple myeloma. Given the effect on plasma cells, some centers have used bortezomib in patients with severe AMR.
COMPLICATIONS
Hyperacute Rejection
• Immediate response due to preformed circulating antibodies to donor antigens (HLA, ABO, and other antigens) that bind the vascular endothelium and initiate the host immunologic response and lead to thrombus formation, inflammatory cell infiltrates, and fibrinoid necrosis of the vessels.8
• Clinically, this results in fulminant allograft failure, although there have been reported cases of successful management with intensive immunosuppression and plasma exchange.
• This complication has become exceedingly rare in recent years because of sensitive screening methods to avoid donors with reactivity to preformed anti-HLA antibodies in potential transplant recipients.
Antibody-Mediated Rejection
• AMR is a more indolent antibody-mediated process that develops over weeks to months and arises from newly developed circulating antibodies to donor antigens.8,9
• No formal diagnostic criteria for AMR have been developed, but the detection of new donor-specific anti-HLA antibodies with allograft dysfunction, pulmonary infiltrates, and evidence of C4d complement deposition and associated pathologic changes in transbronchial lung biopsies help to confirm the diagnosis.9
• Treatment may include a combination of rituximab, plasma exchange, IV immune globulin, and even bortezomib in the most severe cases.
Acute Rejection
• Despite standard three-drug immunosuppressive therapy, many lung transplant patients still experience one or more episodes of acute rejection, especially in the first 6 months after transplantation.8
• Acute rejection is primarily a cell-mediated immune response triggered by recognition of major histocompatibility complex antigens. Pathologic findings include perivascular and/or peribronchiolar lymphocytic infiltrate, with the extent of the inflammation into the surrounding tissue determining the grade of rejection.9
• Most cases of mild acute rejection are asymptomatic and discovered only with surveillance bronchoscopy during the first year following transplantation. More severe cases may present with shortness of breath, nonproductive cough, low-grade fever, and decline in exercise oximetry and spirometry (forced expiratory volume at 1 second [FEV1] decrease by >10%).9
• Examinations may be notable for crackles or rhonchi, with CXR demonstrating nonspecific infiltrates, and blood tests showing leukocytosis.
• Pathologic findings in transbronchial biopsies are the gold standard. Since early stages of acute rejection may be asymptomatic, surveillance bronchoscopies can improve early detection are used by some centers during the first year after transplantation. However, surveillance biopsies still remain controversial, as it does not necessarily improve survival nor decrease the incidence of chronic rejection.9
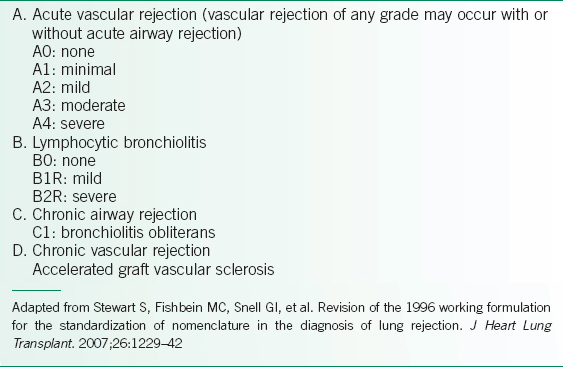
• The International Society for Heart and Lung Transplantation (ISHLT) criteria for acute rejection are listed in Table 29-3 and are based on severity and location.10 Most centers treat acute rejection grades A2 and higher, but practices vary for grade A1 depending on clinical parameters such as lung function or history of prior episodes of acute rejection.
• Initial treatment includes high-dose IV corticosteroids (methylprednisolone, 0.5–1 g IV daily for 3 days). An oral prednisone taper starting at 0.5–1 mg/kg/d over a few weeks may also be used.8,9
• Refractory cases of acute cellular rejection may be retreated with steroids, by alteration of maintenance immunosuppression, with antilymphocyte antibody therapy, or, very rarely, total lymphoid irradiation.8
Chronic Rejection
• Two forms of chronic rejection may be observed: Chronic airway rejection is the most common and is characterized histologically by bronchiolitis obliterans (BO). Chronic vascular rejection manifests by atherosclerosis within the pulmonary vasculature.
• BO is the end result of multifactorial insults to the transplanted tissue. Table 29-4 lists the risk factors linked to chronic rejection.11–19 BO is a fibroproliferative process that begins with lymphocytic infiltration of the submucosa. As the infiltrate migrates into the epithelium, destruction and loss of bronchiolar mucosa follow. Fibroblasts and myofibroblasts are stimulated by this reaction, and subsequently lay down intraluminal granulation tissue. Some airways may remain patent, whereas others are obliterated.
• Chronic rejection manifests as progressive decline in spirometric lung function. Patients may present with worsening dyspnea, cough, wheezing, and low-grade fever. These symptoms may resemble asthmatic bronchitis, usually without improvement after bronchodilators or inhaled corticosteroids.
• Histologic confirmation of BO is difficult, as transbronchial biopsies may not offer adequate tissue for diagnosis.
• BOS is the sine qua non of chronic rejection and is diagnosed based on pulmonary function testing parameters (Table 29-5).8,15,16,19 Since BO primarily affects the small airways, the earliest stages of BOS are detected by a decline in midexpiratory flow rates (forced expiratory flow [FEF] 25–75), followed by a decline in FEV1 and FEV1 to forced vital capacity (FVC) ratio.
• The prevalence of BOS approaches 50% within 3–5 years after lung transplantation.
• Several approaches to treatment include8:
 Intensified or modified immunosuppression (e.g., switch azathioprine to MPA)
Intensified or modified immunosuppression (e.g., switch azathioprine to MPA)
 Initiation of azithromycin three times a week19
Initiation of azithromycin three times a week19
 ATG therapy
ATG therapy
 Extracorporeal photopheresis (ECP)
Extracorporeal photopheresis (ECP)
 Total lymphoid irradiation, rarely used and only in cases of rapidly progressive BOS
Total lymphoid irradiation, rarely used and only in cases of rapidly progressive BOS
 Repeat lung transplantation
Repeat lung transplantation
• Prevention of chronic rejection with empiric three-drug immunosuppression, early and aggressive treatment of respiratory infections, management of gastroesophageal reflux disease (GERD), and regular spirometric testing are important aspects of prevention and management.
TABLE 29-3 CLASSIFICATION AND GRADING OF LUNG ALLOGRAFT REJECTION
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


