23

Lung Transplant Pathology
Lung and heart–lung transplantation are performed in selected patients for the treatment of end-stage pulmonary parenchymal and vascular diseases when life expectancy is significantly shortened (Table 23–1). Worldwide, the most frequently reported indications for lung transplantation are cystic fibrosis and bronchiectasis (about one third of all transplants) and emphysema/chronic obstructive pulmonary disease (another one third of all transplants). Pulmonary fibrosis, particularly idiopathic pulmonary fibrosis, remains a relatively common indication, whereas pulmonary hypertension and lymphangioleiomyomatosis currently make up a smaller percentage of transplants at most centers. Lung transplantation for diffuse bronchioloalveolar carcinoma is done at some centers but remains controversial. Similarly controversial is lung retransplantation for lung transplant recipients who have developed obliterative bronchiolitis or chronic rejection.1–30 Eligibility for lung transplantation depends on (1) general criteria (physiologic age <70, life expectancy <24 months, etc.); (2) no contraindications (HIV infection, liver cirrhosis, active hepatitis B or C infection, active malignancy within the past 2 to 5 years, marrow failure); (3) disease-specific criteria [for example, forced expiratory volume in 1 second (FEV1) < 25%, or PCO2 >55, or resting hypoxemia and/or a deteriorating clinical course for patients with chronic obstructive pulmonary disease]; and (4) consideration of relative contraindications (symptomatic osteoporosis, etc.).
The single-lung transplant is the least difficult procedure technically.13,19 When both lungs must be replaced, bilateral sequential single lung transplants have largely supplanted the original double-lung transplant.17–19,31,32 Transplantation of lobes from living donors has been used at some centers for patients who are too ill to await cadaveric transplantation, particularly patients with cystic fibrosis. Lobar, split lung, and peripheral segmental resection transplantation have been used at some centers to provide lung tissue for small-stature and pediatric recipients.33–35
Two-year survival is in excess of 50% for heart–lung transplant patients, and survival for single-lung transplant patients ranges from greater than 90% at 1 year to greater than 50% at 4 years posttransplant.17–30,36 For living lobar transplantation, survival rates of 70% at 1 year, 54% at 3 years, and 45% at 5 years postsurgery have been reported.33–35 Preoperative diagnosis may have some influence on survival: patients with underlying vascular disease appear to have the worst postoperative courses in some series, in part influenced by the intraoperative use of cardiopulmonary bypass.37,38 In a study of two single-lung transplants per one donor, more numerous fatal complications were noted in patients undergoing left single-lung transplant than right single-lung transplant, speculated to be due to differences in left and right lung harvesting when the retrieval team was different from the transplanting team.39 The Toronto Lung Transplant Program has reported that cardiopulmonary bypass use, body mass index 25 kg/m2, increased immediate postoperative systolic pulmonary arterial pressure, a trend of oxygenation index equal to or greater than 30% from 12 to 14 hours after transplantation, and the Acute Physiology and Chronic Health Evaluation II score predict 30-day mortality and prolonged intensive care unit stay.40
Cystic fibrosis/bronchiectasis |
Emphysema/chronic obstructive pulmonary disease |
End-stage chronic obstructive pulmonary disease |
α1-antitrypsin deficiency |
Pulmonary fibrosis |
Idiopathic pulmonary fibrosis |
End-stage sarcoidosis |
Other end-stage interstitial/parenchymal diseases |
Collagen-vascular disease |
Pneumoconiosis |
Primary pulmonary hypertension |
Pulmonary hypertension secondary to cardiac abnormality |
Lymphangioleiomyomatosis |
Carcinoma |
Retransplantation for chronic rejection/obliterative bronchiolitis |
Recipients experience improvement in symptoms, arterial blood gases, pulmonary function tests, and exercise capacity. When both lungs are replaced, vital capacity, FEV1, and diffusing capacity approach normal, but residual moderate restriction with reduced diffusion capacity is the rule in single-lung recipients with restrictive lung disease.17–19,41,42 Although evidence shows that reinnervation may occur after lung reimplantation in dogs,43,44 stimulation of the transplanted airway at bronchoscopy consistently fails to produce cough in lung transplant patients. This indicates that reinnervation of the transplanted lung does not occur in humans.19,45,46 Despite the loss of pulmonary innervation, the pattern of breathing at rest and during exercise is normal.47 One study has found phrenic nerve dysfunction in 42.8% of lung transplant patients, and these patients required significantly more postoperative days on the ventilator.46 Although studies have shown ciliary activity to be normal in the transplanted single lung, the mucociliary clearance is less than 50% that of the remaining native lung. It is speculated that this discrepancy represents a defect in mucous composition following transplantation.48
Donor lungs must be free of pulmonary disease or significant trauma. ABO matching is necessary to prevent hyperacute rejection and human leukocyte antigens (HLAs) are thought to play a role in acute rejection. Anti-HLA class I antibodies are associated with obliterative bronchiolitis of chronic rejection, and some studies show that HLA mismatching increases the severity of obliterative bronchiolitis of chronic rejection.19,49–51
Immunosuppression protocols vary among institutions and undergo continuing modifications. Lung transplant recipients at Baylor University receive IV methylprednisolone, either cyclosporin A or tacrolimus, and either azathioprine or mycophenolate mofetil prior to their lung transplant surgery.16,19 Currently, about half of lung transplantation patients worldwide have induction therapy with antilymphocyte antibodies, monoclonal anti-CD3 antibody, or anti–interleukin-2-receptor monoclonal antibodies.52–55 Subsequently most patients are maintained on a three-drug regimen. About half of patients are maintained on tacrolimus, and half receive cyclosporin A.53 The second drug may be either azathioprine or mycophenolate mofetil, and the third maintenance drug is steroids. Refractory acute rejection is treated with tacrolimus followed by high-dose steroids or antilymphocyte agents, total lymphoid irradiation or photophoresis. Treatment of high-grade acute rejection with OKT3 may require temporary extracorporeal membrane oxygenation bridging to prevent pulmonary edema. Chronic rejection is treated by modification of the maintenance regimen, addition of inhaled immunosuppressants, and/or total lymphoid irradiation and photophoresis.56–58 Patients are also often maintained on antimicrobial and antipneumocystis prophylaxis. Fungal prophylaxis may be considered in patients colonized with fungal organisms.
Intraoperative or perioperative complications, such as mediastinal hemorrhage or hypotension with acute renal failure, may occur in lung transplant recipients. In single-lung recipients with obstructive disease, there may be a mediastinal shift toward the transplanted lung. Immediately following transplantation, there is a high alveolar-arterial oxygen gradient that gradually subsides.17–19 The transplanted lung may develop diffuse interstitial infiltrates, revealed on a chest radiograph, as a result of fluid overload secondary to reimplantation and interruption of the pulmonary lymphatics.59,60 Within a few days after single-lung transplant, 72% of the blood flow is directed to the transplanted lung; this flow may increase further.17–19
Complications that frequently require histopathologic diagnosis include problems related to the perioperative period including primary graft failure; reimplantation response and ischemia-reperfusion injury; acute, chronic, and rarely hyperacute graft rejection; opportunistic infections; posttransplant lymphoproliferative disorders and their sequelae; pulmonary alveolar proteinosis and other conditions, such as organizing pneumonia and acute respiratory distress syndrome (ARDS), for which the cause may not always be readily apparent; and rare events including recurrence of the original lung pathology, carcinomas, and graft-versus-host disease (Table 23–2).16 As a result of advances in immunosuppressive therapy, surgical technique, and respiratory critical care, opportunistic infection and chronic transplant rejection have superseded anastomotic complications and acute cellular rejection as the major causes of morbidity and mortality in these patients.16–19,61–63
The utility of transbronchial biopsy in diagnosing patients with acute rejection and infection is well established, and surgical wedge biopsy is generally reserved for patients with respiratory failure who require mechanical ventilation. Policies regarding surveillance transbronchial biopsies in patients who are without symptoms vary among institutions.64–67
Primary graft failure |
Reimplantation response |
Ischemia-reperfusion injury |
Hyperacute antibody-mediated rejection |
Acute cellular rejection |
Chronic rejection |
Opportunistic infections |
Posttransplant lymphoproliferative disorders |
Therapy: oxygen, ventilator, drugs |
Recurrence of original lung pathology |
Pulmonary alveolar proteinosis |
Pleural effusions and pleural complications |
Carcinoma |
Graft-versus-host disease |
Primary Graft Failure
Primary graft failure is a devastating postoperative acute lung injury syndrome following lung transplantation. Ischemia-reperfusion injury is a major cause of primary graft failure and occurs within 72 hours after lung transplantation. The histopathologic findings are those of pulmonary edema, diffuse alveolar damage, and sometimes necrosis. Improvements in lung preservation including preservation solutions have resulted in a reduction of the incidence of primary graft failure from 30% of lung transplants to 15% or fewer.40
Reimplantation Response
As noted earlier, patients may experience transient fluid overload with infiltrates, as shown on chest radiograph, due to disruption of the lymphatics and graft ischemia at the time of transplantation.59 The infiltrates decrease within a few days after the pulmonary lymphatics have reestablished systemic connections. This is referred to as the “reimplantation response” and has been demonstrated to produce perfusion, ventilatory, and histopathologic changes in rat lungs after isogeneic transplantation and hilar stripping; these findings confirm that it is not a rejection response.60 Histopathologic findings of reimplantation response have been reported in human heart–lung transplant recipients at autopsy subsequent to early postoperative death.61 These findings consist of dilatation of the peribronchiolar, periarterial, and perivenular lymphatics, pulmonary edema, and mild interstitial and intraalveolar accumulations of neutrophils. Because most of the edema is central, it may not be present on a peripheral biopsy.60
Hyperacute Antibody-Mediated Rejection
Hyperacute rejection is very rare in lung transplant patients and consists of the sudden onset of pulmonary edema and hemorrhage during or immediately after the transplant procedure. Antibodies to class I or II HLAs appear to play a role in the development of this antibody-mediated condition. Most cases are fatal, but successful management with plasmapheresis and antithymocyte globulin has been reported. The histopathologic features include interstitial and intra-alveolar neutrophils, interstitial lymphocytes and alveolar hemorrhage, edema, and hyaline membranes. Septal necrosis, fibrin, platelet thrombi, arteriolar fibrinoid necrosis, and bronchiolar neutrophilic infiltrates with mucosal necrosis may also be seen.68
Acute Cellular Rejection
In the early 1970s, before the advent of cyclosporine, the majority of lung transplant patients died with acute transplant rejection within the first 30 postoperative days. Of 13 single-lung, 1 heart–lung, 1 double-lung, and 3 single-lobe transplants in Veith and Hagstrom’s69 1972 series, 16 died of acute transplant rejection. Today, with the use of modern immunosuppressive therapy, it is distinctly rare for a patient to die of acute lung transplant rejection, and infection and chronic rejection have replaced acute rejection as the leading cause of mortality among lung transplant recipients.16–19,61,62 Timely diagnosis and treatment of acute cellular rejection may prevent chronic rejection (obliterative bronchiolitis or bronchiolitis obliterans syndrome), because of the association of the risk of development of obliterative bronchiolitis with increasing frequency and severity of acute rejection episodes.36,70–75
Approximately 85% of transplant patients have at least one episode of acute cellular rejection during their first postoperative year, but recurrence is common and patients may have episodes of acute rejection at any subsequent time.16,19,76 Unless additional complicating factors (e.g., infection) are present, the clinical findings of acute cellular rejection are almost always reversible with augmented immunosuppressive therapy that typically takes the form of an intravenous bolus of methylprednisolone. In acute cellular rejection, significant clearing of infiltrates should occur within 12 to 24 hours of a steroid bolus, as shown on a chest radiograph.19 Treatment of refractory of acute rejection is as noted above.
The clinical manifestations of acute cellular transplant rejection are relatively nonspecific. They include low-grade fever, decreased gas exchange manifest as a decreased PaO2, reduced exercise tolerance, or small airway obstruction with a drop in percent-predicted FEV1 and percent-predicted maximum midexpiratory flow (MMF).17–19,72 Fluffy hilar radiographic infiltrates may develop comparatively late in the course of a rejection episode. Because infections may also produce these findings, clinical differentiation between the two is difficult. Some of these abnormalities may be more pronounced with acute rejection than with infection. For example, MMF during acute rejection is significantly lower than MMF during infection and may help to clinically distinguish between these two processes.72 Likewise, serum elevations of soluble interleukin-2 receptors released by activated T lymphocytes may be greater with acute rejection than with infection.76 In single-lung transplants, decreased blood flow to the transplanted lung on quantitative radionuclide scan is a sensitive, but nonspecific, finding during rejection.19 Ultimately, diagnosis of acute rejection depends on (1) clinical improvement with empiric immunosuppressive therapy after infection has been reasonably ruled out on lung biopsy and bronchoalveolar lavage (BAL), or (2) the finding of histopathologic evidence of acute rejection on lung biopsy.
Pathology of Acute Cellular Transplant Rejection
Studies in both humans and animals before the advent of modern immunosuppressive therapy, particularly cyclosporine, or on untreated animal models more recently have provided information on the histopathology of acute cellular lung transplant rejection.77–86 Based on human and animal studies in the early 1970s, Veith et al69,79,83,87 categorized acute lung transplant rejection into three histopathologic patterns: (1) classic, with perivascular lymphocytic infiltrates and fibrinous alveolar exudates; (2) atypical, with alveolar exudates only; and (3) vascular, with perivascular infiltrates only. In rats with untreated lung transplant rejection, Prop et al88–90 identified the following progressive histopathologic stages: (1) latent; (2) vascular, with perivenous lymphocytic infiltrates, followed by peribronchial and periarterial infiltrates; (3) alveolar, with interstitial and intraalveolar infiltrates of lymphocytes and macrophages, followed by intraalveolar exudate; and (4) destructive, with necrosis of the alveolar wall.
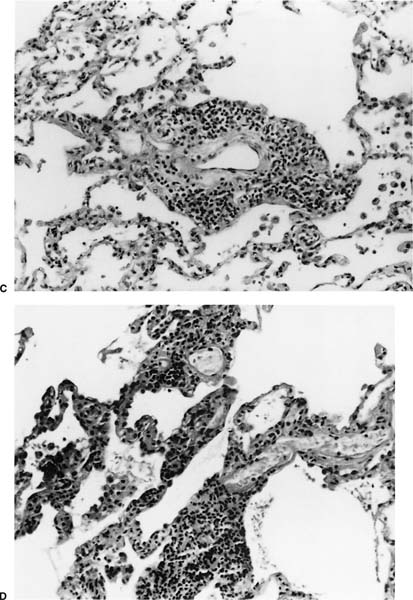
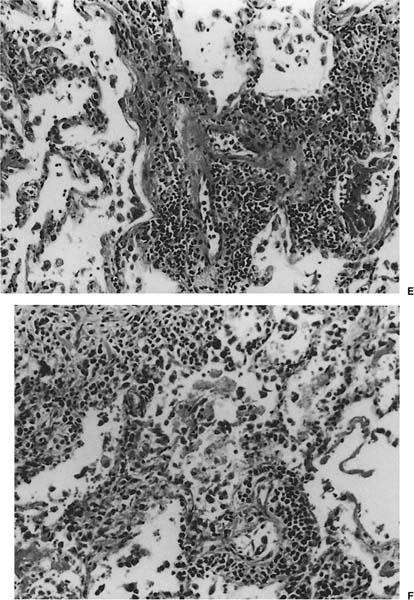
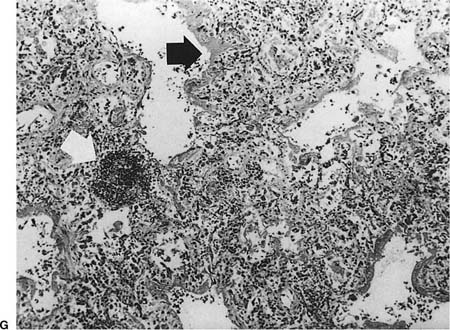
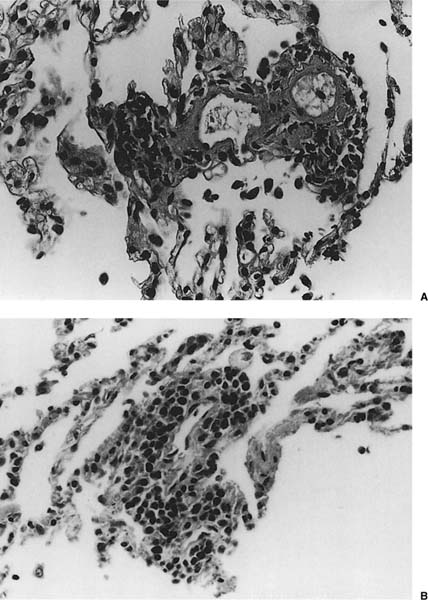
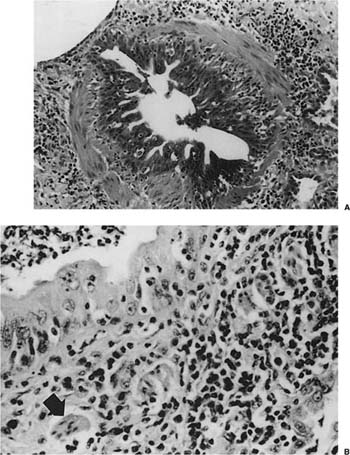
FIGURE 23–1 Acute rejection. (A) Minimal: Perivascular lymphocytic infiltrates are present but scanty and no more than two or three cells thick. There is no noticeable expansion of the adventitia. When viewed at low power, this finding is subtle and easily overlooked [hematoxylin and eosin (H&E), × 400]. (B) Mild: There is slight expansion of the perivascular adventitia by lymphocytic infiltrate without extension into adjacent septa. In this example, the lymphocyte infiltrate is only modestly more dense than in minimal rejection (H&E, × 400). (C) Mild: There is obvious expansion of the perivascular adventitia by lymphocytes, although extension into adjacent alveolar septa is not conspicuous at this point (H&E, ×150). (D) Moderate: Extension of lymphocytic infiltrate into the alveolar interstitium accompanies the perivascular lymphocytic infiltrates (H&E, ×150). (E) Moderate: Increasingly intense perivascular and interstitial inflammatory infiltrates, with modest spillage of inflammatory cell into airspaces (H&E, ×150). (F) Severe: Diffuse perivascular and interstitial infiltrates are accompanied by fibrinous exudate, hyaline membranes, intraalveolar macrophages, and sloughing of pneumocytes (H&E, ×150). (G) Severe: Acute rejection consisting of diffuse alveolar damage with hyaline membranes (dark arrow), accompanied by perivascular lymphocytic infiltrates (light arrow) (H&E, ×60).
No histopathologic finding on transbronchial biopsy is completely pathognomonic of acute cellular transplant rejection because of the potential overlap of these findings with other processes, particularly infection. Polymorphous infiltrates of predominantly mature and activated lymphocytes admixed with plasmacytoid lymphocytes, plasma cells, and histiocytes around veins, venules, arterioles, and small to medium-size arteries are the most consistent and relatively specific feature of acute rejection (Table 23–3).16,70,73,91–97 The number of vessels involved and the intensity of the perivascular infiltrates increase with the severity of rejection, and grading systems have been proposed on this basis.73 A working formulation to standardize the grading of lung transplant rejection was proposed by a study group of the International Society for Heart Transplantation, which was followed by a revised working formulation (Table 23–4, Fig. 23–1A-F).96,98 Eosinophils and occasionally neutrophils may also be present in the infiltrates. They become more prominent with increasing severity of rejection. Lymphocytic endovasculitis may be seen in association with the perivascular infiltrates; it likewise becomes more prominent with increasing histopathologic grade of rejection. Subsequent to therapy, follow-up biopsies may be (1) unchanged; (2) show diminished perivascular infiltrates with less prominence of the activated lymphocytes; or (3) show complete resolution of the infiltrates and, occasionally, small hemosiderin deposits around the blood vessels.
In minimal and mild rejection, the lymphocytic infiltrates are well circumscribed around vessels. Overflow of the perivascular infiltrates into the surrounding alveolar interstitium occurs with more severe grades of rejection (moderate and severe). Minimal perivascular lymphocytic infiltrates may be seen around one or a few sampled blood vessels in surveillance biopsy from a patient who is asymptomatic. These infiltrates presumably represent subclinical rejection. In these cases, clinical follow-up and perhaps a repeat biopsy at a later date may be useful to determine their significance.
Inflammation of the airways may or may not be associated with the perivascular infiltrates in all grades of acute rejection, and grading of airway inflammation was added to the revised working formulation (Table 23–5). Typically there are peribronchiolar and intraepithelial lymphocytic infiltrates involving the terminal and respiratory bronchioles that are similar to those involving the blood vessels. The bronchi may also have intraepithelial and subepithelial lymphocytic infiltrates. These are often accompanied by histiocytes and plasma cells.
Any persistence of airway inflammation after perivascular lymphocytic infiltrates of acute rejection have responded to immunosuppressive therapy may be the basis of the airway injury referred to as lymphocytic bronchitis/bronchiolitis.99 Injury to the airways resulting from prolonged or recurrent acute rejection is thought to be a contributing factor to the obliterative bronchiolitis seen in chronic rejection and bronchiectasis.36,61,70–75,93,100
Other findings that may be present in severe acute cellular rejection include organizing pneumonia (which may be seen with ongoing acute cellular rejection or as a result of an antecedent resolving acute rejection), diffuse alveolar damage with hyaline membranes and fibrinous exudates (Fig. 23–1G), organizing diffuse alveolar damage with an organizing pneumonia pattern, intraalveolar hemorrhage, and intraalveolar macrophages and neutrophils. Necrotizing vasculitis and parenchymal necrosis may sometimes occur in severe rejection.16,73,96
Although perivascular lymphocytic infiltrates are comparatively specific for acute cellular rejection, all of the histopathologic features of acute cellular rejection, including perivascular lymphocytic infiltrates, interstitial lymphocytic infiltrates, bronchiolar and bronchial inflammation, organizing pneumonia, and diffuse alveolar damage, may potentially be seen in other conditions to which the transplant patient is susceptible, particularly infection. Obliterative bronchiolitis may sometimes also be present in a biopsy with features of acute rejection and may represent underlying chronic rejection or superimposed infection. Relatively pure perivascular lymphocytic infiltrates in the absence of pathologic or clinical evidence of infection or other processes can generally be interpreted as acute rejection. Perivascular lymphocytic infiltrates occur more frequently and more intensely in rejection than in cytomegalovirus (CMV) pneumonitis, as does endotheliitis. In acute rejection, the lymphocytic infiltrates are centered around the vessels with extension into the interstitium, whereas CMV pneumonitis shows predominantly interstitial inflammation involving associated vessels.97 Perivascular infiltrates of atypical lymphocytes or immunoblasts may occur in Epstein-Barr virus (EBV)–associated posttrans-plant lymphoproliferative disorders and may resemble the perivascular lymphocytic infiltrates of acute rejection at low power. These can be ruled out by the atypia of the lymphoid cells, by the detection of monoclonality or EBV infection of the lymphoid cells, and by a response to immunosuppressive therapy that is diametrically opposite to that of acute cellular rejection (Table 23–6).
In most cases: |
Perivascular lymphocytic infiltrates |
With or without bronchial/bronchiolar lymphocytic infiltrates |
In severe cases: |
Perivascular lymphocytic infiltrates with adjacent interstitial lymphocytic infiltrates |
Diffuse alveolar damage |
Organizing pneumonia |
Intraalveolar hemorrhage |
Intraalveolar macrophages and neutrophils |
Necrotizing vasculitis and parenchymal necrosis |
Grade | Histologic Findings |
|---|---|
A0 | No mononuclear cells |
A1 (minimal) | Lymphoplasmacytic infiltrates; two to three cells thick in perivascular connective tissue |
A2 (mild) | Lymphoplasmacytic infiltrates, macrophages, eosinophils; >3 cells thick around venules and arterioles, “endotheliitis” or infiltration of endothelium may be present |
A3 (moderate) | Lymphoplasmacytic infiltrates, macrophages, eosinophils, occasional neutrophils; extension into perivascular and peribronchiolar alveolar septa and airspaces |
A4 (severe) | Lymphoplasmacytic infiltrates, macrophages, eosinophils, neutrophils; diffuse infiltrates (perivascular, interstitial, alveolar) with alveolar pneumocyte injury and necrosis, hyaline membranes, hemorrhage, neutrophils, parenchymal necrosis, infarction, necrotizing vasculitis |
Grade | Histologic Findings |
|---|---|
B0 | No lymphoplasmacytic infiltrates |
B1 (minimal) | Rare lymphoplasmacytic cells in bronchial or bronchiolar submucosa |
B2 (mild) | Circumferential lymphoplasmacytic infiltrate with or without occasional eosinophils in bronchial or bronchiolar submucosa; a few intraepithelial lymphocytes may be present |
B3 (moderate) | Dense circumferential lymphoplasmacytic infiltrates with numerous intraepithelial lymphocytes, lymphocyte satellitosis, and epithelial cell apoptosis |
B4 (severe) | As above plus features with epithelial detachment, ulceration, and/or fibrinopurulent exudate |
BX (not gradable) | Grade cannot be assigned due to sampling or technical problem, or other existing pathology such as infection |
| Features Useful in Diagnosis |
Acute rejection | Predominantly vessel-centered with secondary involvement of interstitium; endovasculitis may be prominent; responds to immunosuppression |
Infections (predominantly viral) | Predominantly interstitial with secondary involvement of vessels; identification of organism by histopathology, culture, or special studies; responds to antimicrobial therapy |
Posttransplant lymphoproliferative disorder | Atypical or frankly lymphomatous infiltrate; mitoses and necrosis may be present; special studies for clonality or Epstein-Barr virus; follows augmented immunosuppression; responds to reduction of immunosuppression |
However, not only can infection sometimes mimic rejection, infection may occur simultaneously with acute cellular rejection, and treatment for both may be warranted.16 Organisms may be readily apparent on biopsy or accompanying BAL fluids. Special techniques to identify organisms, such as cultures, immunohistochemistry, in situ hybridization, or polymerase chain reaction (PCR) amplification, may be helpful in cases where infection is suspected but organisms are not identifiable on routine stains. In equivocal or complicated cases, familiarity with the patient’s clinical history and consultation with the lung transplant pulmonologist are important in the final interpretation of histopathologic findings.
Diagnosis of acute cellular rejection by transbronchial biopsy requires adequate sampling of diagnostic blood vessels.93,96 In dogs that had undergone lung transplant, Koerner et al101 found that open biopsy was superior to transbronchial biopsy in diagnosing acute rejection; however, in human patients transbronchial biopsy is preferable because it is a less invasive procedure.
Sensitivity of the transbronchial biopsy increases with the number of specimens obtained; it also depends on the severity of the rejection. A minimum of five biopsies has been recommended for the diagnosis of acute rejection,96 but it is not certain that this number permits reliable determination of the maximum grade of rejection.73 Tazelaar et al102 conducted a study of single-lung transplants in dogs, in which they examined the entire lung subsequent to a standardized series of transbronchial biopsies. They found that five pieces of lung tissue were needed per transbronchial biopsy to yield a sensitivity of 92% when mild acute rejection is present, and that three pieces of lung tissue are needed to yield an equivalent sensitivity when moderate or severe acute rejection is present. Higenbottam et al8,91,92,94,95 performed a minimum of 8 to 12 biopsies per bronchoscopy, and processed and examined 50 sections per biopsy; they obtained a sensitivity of 84% in diagnosing acute rejection by transbronchial biopsy.
Clinically significant acute cellular rejection that obviously requires therapy should, in most cases, be readily apparent on fewer biopsy samples. There are rare situations in which a patient has clinical deterioration suspicious for rejection, but biopsy does not yield a definitive diagnosis of rejection. In these cases, empiric treatment for rejection can usually be instituted with close monitoring of response, provided that biopsy and BAL have reasonably ruled out infection. Extensive sampling, which on multiple levels detects only a rare vessel with minimal infiltrates in a patient without symptoms or signs of rejection, may not be worth the expense or, more importantly, the risk of such complications as bleeding from the biopsy procedure. The possible benefits of treatment for rejection in a case like this, including the possible avoidance or delay of chronic rejection, must be weighed against the risks of augmented immunosuppression including infection.
In heart–lung transplant recipients, rejection of the heart allograft and rejection of the lung allografts occur independently. Rejection of the lung allografts is observed more frequently than rejection of the cardiac allograft.103–105 Therefore, biopsy of the endomyocardium is not useful in assessing rejection of the lung allografts in these patients.
Airway Inflammation
Predominantly Lymphocytic
Lymphocytic bronchitis/bronchiolitis (LBB) consists of intraepithelial and subepithelial mononuclear infiltrates of large and small airways (Fig. 23–2).99,106,107 Other features of LBB may include mononuclear infiltrates in the smooth muscle, subepithelial granulation tissue, squamous metaplasia and epithelial apoptosis, and necrosis with occasional neutrophils. LBB was given a separate category, “active airway damage without fibrous scarring,” in the original working formulation because progression to obliterative bronchiolitis was not believed to be inevitable. LBB is often identified after an episode of acute rejection that has a component of airway inflammation. Therefore, LBB may represent the persistence of airway-centered rejection subsequent to treatment of acute rejection. In Yousem’s99 series, 39% of patients with LBB developed obliterative bronchiolitis. The presence of submucosal granulation tissue and bronchiolitis were observed about twice as often in patients who eventually developed obliterative bronchiolitis than in those who did not. LBB may stabilize or improve with augmented immunosuppression. As with other inflammatory infiltrates in lung transplant patients, infection and other causes of inflammation should be considered in the differential diagnosis of LBB.
FIGURE 23–2 Lymphocytic bronchitis/bronchiolitis. (A) Lymphocytes and plasma cells with an occasional neutrophil infiltrate the submucosa and mucosa of a bronchiole. The bronchiolar epithelium shows reactive changes and metaplasia. No submucosal fibrous scarring is present. Adjacent blood vessels do not show perivascular lymphocytic infiltrates (H&E, × 100). (B) Infiltration of lymphocytes, plasma cells, and occasional neutrophils in the epithelium and submucosa and around smooth muscle bundles (arrow) of a bronchus. The epithelium shows reactive changes. This patient was later found to have bronchiolitis obliterans (H&E, ×600).
Predominantly Neutrophilic
Acute bronchitis/bronchiolitis (ABB) consists of infiltration of bronchi and bronchioles by neutrophils. In the series of Ohori et al,108 five clinicopathologic categories were identified: harvest injury, acute cellular rejection, bronchiolitis obliterans syndrome, infection, and other manifestations. Outcome and additional histopathologic findings depend on the etiology. Luminal dilatation, mucus plugging, and granulation tissue formation may also be seen in these cases.
Pulmonary Thromboemboli
The extent to which pulmonary thromboemboli are a significant complication of lung transplantation has not been established. In a study of 126 autopsies of lung and heart–lung transplant recipients, Burns and Iacono109 found that the prevalence of pulmonary emboli was 36.4% in their early posttransplant group (1–30 days), 20.0% in their intermediate posttransplant group (31–365 days), and 23.8% in their late posttransplant group (>365 days).
Chronic Rejection
Obliterative Bronchiolitis or Bronchiolitis Obliterans Syndrome
Obliterative bronchiolitis or bronchiolitis obliterans syndrome is the most significant manifestation of chronic transplant rejection in long-term lung transplant survivors and is the most frequent cause of death in long-term survivors.2,3,6,7,16,61,62,74,106,110–118 Obliterative bronchiolitis is an inflammatory process that results in fibrosis of the terminal (membranous) bronchioles and respiratory bronchioles with eventual occlusion of these small airways. Since transbronchial biopsy is not a sensitive way to diagnose obliterative bronchiolitis, bronchiolitis obliterans syndrome (BOS) has been described to allow clinical diagnosis of chronic rejection. BOS is defined clinically as a greater than 20% decrease from baseline in the FEV1. A new stage of “potential BOS” or BOS 0-p has been designated by a consensus panel of the International Society for Heart and Lung Transplantation for the purpose of identifying patients at high risk of BOS when intervention may be more successful (Table 23–7).119–122
Stage | Clinical Feature |
|---|---|
BOS 0 | No significant abnormality; FEV1 80% of reference value |
BOS 0-p (potential) | 10 to 19% decline in FEV1 compared with peak FEV1 |
BOS 1 (mild) | FEV1 66 to 80% of reference value |
BOS 2 (moderate) | FEV1 51 to 65% of reference value |
BOS 3 (severe) | FEV1 50 or less of reference value |
Subcategories: a = without pathologic evidence of obliterative bronchiolitis; b = with pathologic evidence of obliterative bronchiolitis.
Patients with BOS present with insidious onset of cough and dyspnea that relentlessly progresses to irreversible disabling airflow obstruction or death. Augmented immunosuppressive therapy may sometimes slow or arrest the progression of the airflow obstruction.123,124 In early series, as many as 30 to 50% of the heart–lung recipients developed bronchiolitis obliterans at 3 months or longer after transplant; the mortality rate was 50%.2,3,6,7,61 Although aggressive treatment of acute rejection detected early by transbronchial biopsy has been reported to result in dramatic drops in incidence of chronic rejection,70
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree