Lung Transplant and Tracheal Stent
Presentation
A 56-year-old truck driver has been incapacitated for several years because of chronic obstructive pulmonary disease (COPD). The patient reports having shortness of breath while walking from the bedroom to the bathroom. Recently, he was prescribed oxygen at 3 L/min to use continuously.
Past medical history includes hypertension and moderate peripheral vascular disease. The patient has a 40-pack-per-year history of smoking. On physical examination, the vital signs are stable; oxygen saturation is 91% on 3-L nasal cannula. There are decreased breath sounds and fine crackles bilaterally. Diagnostic tests include a chest x-ray, which demonstrates hyperinflated lungs compatible with COPD. There are no infiltrates or masses. The heart size is normal, and there are no pleural effusions. Pulmonary function tests reveal a forced expiratory volume in 1 second (FEV1) of 0.67 L and a forced vital capacity (FVC) of 1.86 L.
Discussion
Medical therapy for end-stage COPD consists of bronchodilators, expectorants, pulmonary care, steroids, and prevention and treatment of pulmonary infection. Lung transplantation should be considered in patients for whom lifestyle is severely restricted and life expectancy is diminished.
Lung transplantation is a treatment option for many end-stage pulmonary diseases. It is specifically performed in patients who have a life expectancy of less than 1 to 2 years and are severely debilitated. Single-lung transplantation is performed in patients with obstructive or interstitial lung disease, whereas bilateral sequential lung transplantation is performed in patients with infectious pulmonary processes, such as cystic fibrosis and bronchiectasis, and for young patients with emphysema. Combined heart-lung transplantation is reserved for end-stage lung and heart disease.
Candidates for lung transplantation are patients with diminished exercise capacity, poor pulmonary function, and in most cases, oxygen dependency. Contraindications for lung transplantation include acute infection; malignancy; irreversible dysfunction of renal, hepatic, cardiac, or nervous system; and psychiatric conditions such as addiction or noncompliance. Relative contraindications to lung transplantation include ventilator dependency, prior thoracic surgery, and advanced age. In a candidate for single-lung transplantation, the side chosen to transplant is the one that has the worst function as measured by ventilation-perfusion mismatch.
Case Continued
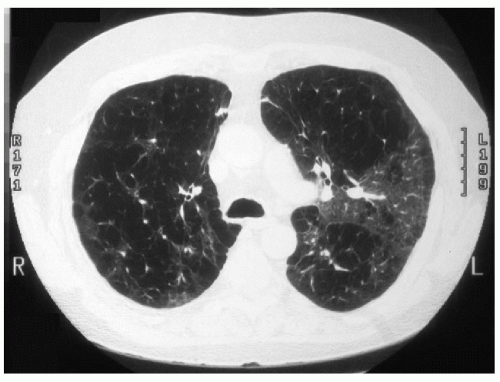
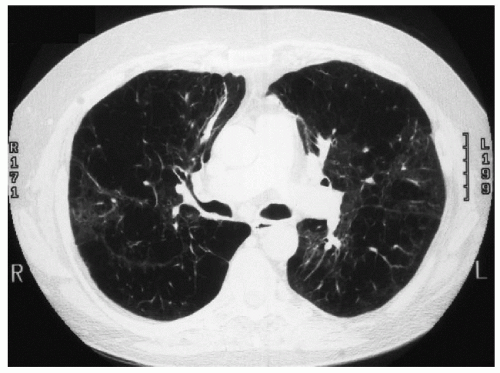
Assessment of the patient by the lung transplantation team includes an echocardiogram, cardiac catheterization, ventilation-perfusion scan, pulmonary function tests, bronchoscopy, and surveillance for infection. The patient is referred to a pulmonary rehabilitation program to maximize his physical condition, and he is provided with information about transplantation procedure, immunosuppression, and risks. The following computed tomography (CT) scans are obtained in the preoperative evaluation.
Case Continued
After 4 months, the transplantation coordinator receives a call that a lung is available for this patient. Most organ donors are victims of trauma or cerebral catastrophe. Before an organ can be accepted for harvesting, brain death must be established. An acceptable donor should be younger than 55 years of age and must have ABO compatibility with the recipient. Donor operation coordinates multiple teams for multiple organ harvesting. To be an acceptable organ, the lung should not have incurred direct trauma, contusion, or hemorrhage. On 100%
fraction of inspired oxygen (FIO2) and positive end-expiratory pressure (PEEP) of 5 cm, the partial pressure of arterial oxygen (PaO2) should be at least 300 mm Hg. The chest x-ray of the donor must be clear of masses and infiltrates, serology of the donor [human immunodeficiency virus (HIV), hepatitis C, and cytomegalovi – rus (CMV)] is checked, and any history of previous lung disease is ascertained. The size match should be within 15% to 20% of the calculated total lung capacity (TLC) of the patient. Measurements of the circumference as well as vertical and horizontal lengths are also used in the assessment of the fit for the recipient. Finally, bronchoscopy at the time of harvesting is essential to exclude purulent bronchial secretions and aspiration, either of which contraindicates use of the lung as an acceptable donor organ.
fraction of inspired oxygen (FIO2) and positive end-expiratory pressure (PEEP) of 5 cm, the partial pressure of arterial oxygen (PaO2) should be at least 300 mm Hg. The chest x-ray of the donor must be clear of masses and infiltrates, serology of the donor [human immunodeficiency virus (HIV), hepatitis C, and cytomegalovi – rus (CMV)] is checked, and any history of previous lung disease is ascertained. The size match should be within 15% to 20% of the calculated total lung capacity (TLC) of the patient. Measurements of the circumference as well as vertical and horizontal lengths are also used in the assessment of the fit for the recipient. Finally, bronchoscopy at the time of harvesting is essential to exclude purulent bronchial secretions and aspiration, either of which contraindicates use of the lung as an acceptable donor organ.
▪ Surgical Technique: Donor
After bronchoscopy of the donor, the chest is opened through a median sternotomy, and the lung is inspected for adhesions, contusions, and hemorrhage. The lung is mobilized; the trachea is dissected, and a tape is placed around it. The superior vena cava (SVC) is mobilized, and a tape is placed around it. After the abdominal organ team completes their dissection, 30,000 units of heparin are administered intravenously to the patient. In addition, 500 υg of prostaglandin E1(PGE1) are injected directly into the pulmonary artery. PGE1 causes systemic hypo – tension, at which time the aorta is cross-clamped and cardioplegia is administered for cardiac preservation. Cold pulmoplegia (3 L of Euro-Collins solution) is delivered into the pulmonary artery. The effluent is vented through the left atrial appendage and the inferior vena cava at the level of the diaphragm. Ice and cold saline are placed in the chest cavity to provide topical cooling. Simultaneously, the SVC is ligated (to prevent warm blood from entering the right heart), and the innominate artery and vein are stapled to facilitate exposure of the trachea. Ventilation is maintained with 100% oxygen, keeping the lungs gently inflated. After the heart and lungs are preserved, the endotracheal tube is pulled back, ventilation is maintained to keep the lungs at least two thirds inflated, and the trachea is stapled and transected. The heart is excised, taking care to leave a sufficient left atrial cuff with sufficient length on the pulmonary veins. The pericardium is cut from the diaphragm, behind the hilum, and continuing beneath the trachea. Dividing the posterior wall of the remaining left atrial cuff separates the pulmonary veins, and the pulmonary artery is bisected at the bifurcation. The left main bronchus is doubly stapled and transected, keeping both donor lungs inflated. The donor lung is placed in a sterile bag, ice and cold saline are placed into the bag, and the bag is tied securely. After double bagging, the lung is placed in a cooler filled with ice and taken expeditiously by the harvesting team to the recipient hospital. Ischemia time begins with the cross-clamping of the aorta and infusion of pulmoplegia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree