6 Lung Infections
Lower respiratory tract infections constitute a leading cause of morbidity and death worldwide.1,2 Included in this category of infections are bronchitis and bronchiolitis, community-acquired and nosocomial pneumonias, and pneumonias in the immunocompromised patient. A relatively small percentage of these infections come to the attention of the surgical pathologist, because most are diagnosed in the microbiology laboratory. Nevertheless, as summarized in Box 6-1, the anatomic pathologist can play a pivotal role in the diagnosis of lung infections by identifying reaction patterns in tissue, and sometimes in the identification of an organism that microbiologic techniques fail to detect.3 Despite significant advances in laboratory techniques, culture diagnosis is not always possible; the organism may not reproduce in culture, a culture study may not have been requested, or the culture technique may have failed for any of various technical reasons. Even when culture is successful, the time frame for diagnostic purposes may not be clinically useful, or the culture result, in the absence of an expected tissue response, may not permit distinction of pathogens from innocent bystanders, be they colonizers or contaminants. For all of these reasons, the pulmonary infection for which biopsy is performed often is one that has eluded standard microbiologic techniques, has not responded to empirical therapy, or requires morphologic analysis for clarification of a critical aspect of the differential diagnosis. In these situations, the diagnostic pathologist is indispensable,4,5 if not for providing an immediate report intraoperatively (by frozen section or cytologic imprints or smears), then for dramatically improving diagnosis turnaround time with the use of newer rapid tissue-processing systems6 (Table 6-1).
Box 6-1 Role of the Diagnostic Pathologist
Modified from Watts J, Chandler F. The surgical pathologist’s role in the diagnosis of infectious disease. J Histotechnol. 1995;18:191–193.
Table 6-1 Diagnostic Tools of the Pathologist
| Activity | Objective |
|---|---|
| Pre-/intra-/postoperative consultation | Information exchange and strategies |
| Gross examination | Tissue handling and triage |
| Histopathologic examination | Organism morphology; cytopathic effect; host response |
| Histochemical stains | Detection and morphologic detail |
| Immunohistochemical stains | Detection of organisms; confirmation of genus/species |
| Electron microscopy | Selective use for virus, fungi, parasites, and bacteria |
| Molecular techniques: in situ hybridization, polymerase chain reaction | Sensitive and specific detection/identification of nonculturable organisms; stain-negative cases |
| Report | Clinicopathologic and microbiologic correlation |
Diagnostic Tools and Strategies
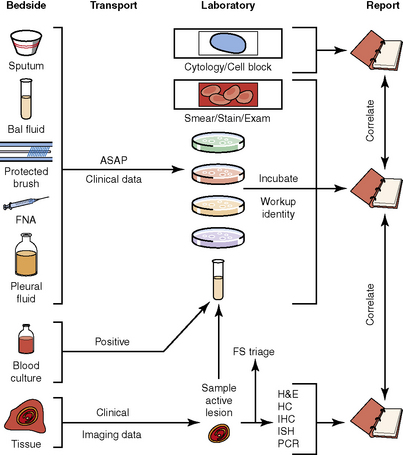
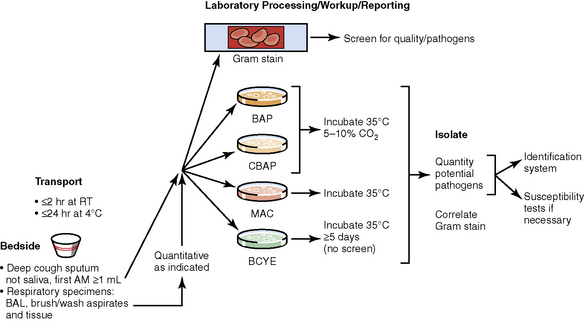
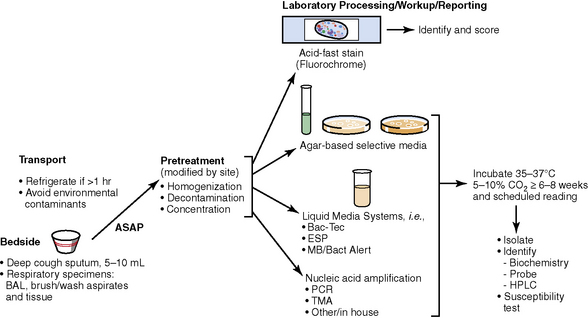
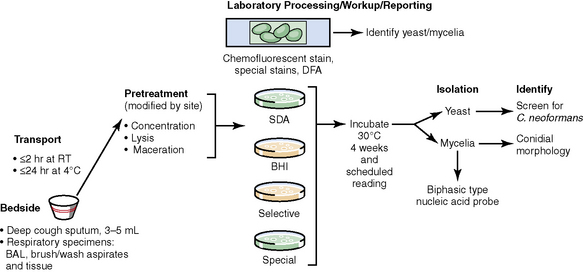
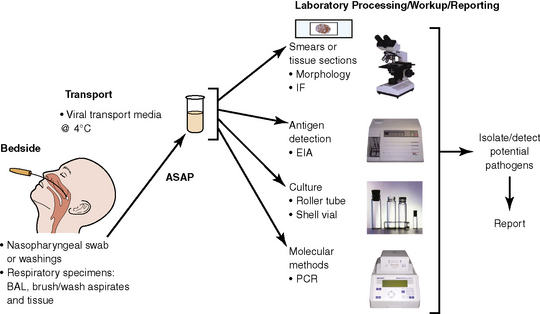
The history of the field of pathology is intertwined with the discovery of pathogenic bacteria and the development of the science of microbiology.7 Today, pathologists and microbiologists approach the diagnosis of infection with techniques and methods that share some aspects but have important differences.8 The surgical pathologist and the cytopathologist are in a position to apply the tools of both disciplines to achieve a clinically relevant diagnosis by correlating the histopathologic or cytopathologic examination findings with data obtained using microbiology techniques (Table 6-2). Unfortunately, the diagnostic workup and reporting of biopsy findings in surgical or cytopathology departments and those in the microbiology laboratory typically run along nonintersecting paths, often without one group knowing (or acknowledging) the findings of the other. An interdisciplinary approach that is based on mutual understanding and communication would seem to be a logical, if not ideal, scenario for optimal clinical management.9 Our concept of an integrated morphologic and microbiologic approach is presented schematically in Figure 6-1, and with greater detail for specific situations in which bacterial (Fig. 6-2), mycobacterial (Fig. 6-3), fungal (Fig. 6-4), or viral (Fig. 6-5) pathogens are suspected.
Table 6-2 Diagnostic Tools of the Microbiologist
| Activity | Objective |
|---|---|
| Pre-/intra-/postoperative consultation | Information exchange and strategies |
| Direct visualization (smears and imprints) | Rapid detection |
| Culture | Identification of genus and species; susceptibility studies |
| Antigen detection | Rapid identification |
| Serologic testing | Specific antibody response |
| Molecular techniques | Sensitive and specific detection/identification |
| Report | Traditional versus interpretive format |
In current medical practice, identification of the genus or species of an infectious organism can have important prognostic and therapeutic implications. Because histopathologic examination alone rarely provides this information, the findings should always be correlated with results of cultures. Accordingly, foresight is required on the part of the intraoperative pathologist in obtaining and properly handling tissues for culture.10 The correlation of the morphologic and microbiologic data can be facilitated in the surgical pathology report by appending a comment that seeks to enhance the morphologic diagnosis by suggesting a specific etiologic disorder or agent, considerations for the differential diagnosis, or additional workup with culture, serology, or molecular studies. In certain situations, it is also appropriate to include the preliminary results of microbiology stains and cultures, and to correlate this information with the morphologic findings whenever possible.
Knowledge of the Clinical Setting
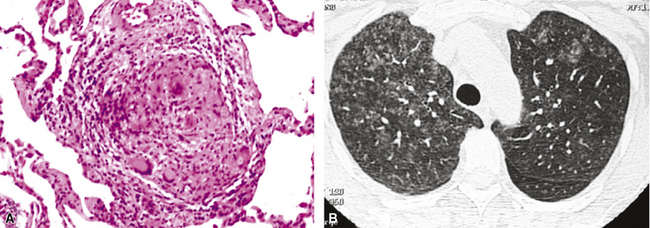
Identification of risk factors and determination of the immune status of the patient are of primary importance, because these parameters typically influence the spectrum of histopathologic changes and the type of etiologic agents and pathogen burden.11–16 Also, because the degree of immunosuppression often influences the burden of organisms, different efforts may be required to identify the pathogen. For example, organisms are less often found in lung tissues from patients with normal or near-normal immunity. In this setting, cultures, serologic studies, and epidemiologic data must be relied on to provide the diagnosis.17 By contrast, persons infected with the human immunodeficiency virus (HIV) in whom the acquired immunodeficiency syndrome (AIDS) or Mycobacterium avium infection develops typically manifest poorly formed granulomas, or simply histiocytic infiltrates, despite an overabundance of organisms identified by tissue acid-fast stains. Pneumocystis organisms may be easily identified in patients with AIDS, who manifest diffuse alveolar damage accompanied by abundant, foamy alveolar casts but when immunosuppression is less severe (such as that produced by corticosteroids therapy for arthritis), the morphologic features can be less typical, and the organisms sparse. The relationship among the level of immunity, burden of organisms, and patterns of disease is illustrated for cryptococcosis in Figure 6-6.
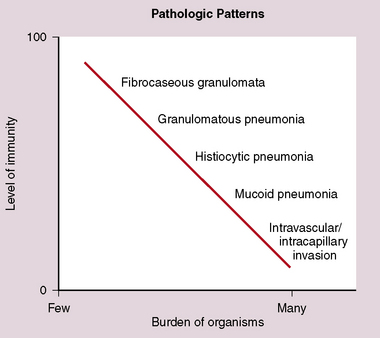
Figure 6-6 Cryptococcosis: Correlation of pathologic patterns with immunity level and organism burden.
(Data from Mark EJ. Case records of the Massachusetts General Hospital. N Engl J Med. 2002;347:518–524.)
In the immunocompromised patient, one must also consider a broader differential diagnosis. In addition to infection, other disorders come into consideration such as pulmonary involvement by pre-existing disease, drug-induced and treatment-related injury, noninfectious interstitial pneumonias, malignancy, and new pulmonary diseases unrelated to the patient’s immunocompromised state, such as aspiration, heart failure, and pulmonary embolism. When immunosuppression is intentional, as in transplant recipients, unique additional challenges come into play, such as transplant rejection, graft-versus-host disease, and Epstein-Barr virus (EBV)-associated lymphoproliferative disorders. Immunosuppressed persons are at risk for multiple simultaneous infections, so when one organism is found, a careful search for others is always warranted (Fig. 6-7).
A number of well-characterized genetic disorders of immunity and cellular function are known to predispose affected persons to lung infection.18–21 Cystic fibrosis bears special recognition in this context because it is associated with reproducible patterns of lung disease and susceptibility to a wide spectrum of infectious organisms. This geneticdisease of autosomal recessive inheritance involves mutation of the CFTR gene that affects the ability of epithelial cells to effectively transport chloride and, secondarily, water across cell membranes. As a result, many organs, including the lungs, develop excessively viscous mucous secretions, which cannot be cleared effectively from the airways. In the lung, retention of such secretions leads to progressive and widespread bronchiectasis with airway obstruction that in turn paves the way for recurrent infection (Fig. 6-8). Bacterial organisms commonly isolated include Pseudomonas aeruginosa (both mucoid and nonmucoid strains), Haemophilus influenzae, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Burkholderia cepacia complex, Stenotrophomonas maltophilia, and Achromobacter xylosoxidans.22 Polymicrobial infections are not uncommon, and some of these pathogens, especially certain subspecies within the B. cepacia complex, are linked to an adverse prognosis.23 Cystic fibrosis also is a risk factor for non-tuberculous mycobacterial infection and allergic bronchopulmonary fungal disease, and the condition is potentially exacerbated by superimposed viral infections.24–27
Pattern Recognition
Knowledge of the radiologic pattern of infectious lung disease in a given patient often helps to narrow the scope of the differential diagnosis.28,29 Patterns of lung infection seen on high-resolution computed tomography (HRCT) typically are dominated by increased attenuation (opacity). Such opacities may occur as one or more localized densities (nodule, mass, or localized infiltrate) or as more extensive infiltrates referred to as either ground-glass opacities (attenuation that allows underlying lung structures to be visible) or consolidation (attenuation that overshadows underlying structure).30 Review of the chest imaging studies with the radiologist can be very helpful in arriving at a clinically relevant diagnosis. Correlating these data with the clinical history and pace of the disease under scrutiny (acute, subacute, chronic) allows a more accurate interpretation of the observed histopathologic pattern of disease in the tissue (Fig. 6-9). Fortunately, the recognized histopathologic patterns of lung infection are fairly limited (airway disease, acute lung injury, cellular infiltrates, alveolar filling, and nodules), and these typically correlate with a particular group of organisms (Table 6-3).
Table 6-3 Histopathologic Patterns and Most Agents of Pulmonary Infection
| Pattern | Most Common Agent(s) |
|---|---|
| Airway disease | |
| Bronchitis/bronchiolitis | Virus; bacteria; Mycoplasma |
| Bronchiectasis | Bacteria; mycobacteria |
| Acute exudative pneumonia | |
| Purulent (neutrophilic) | Bacteria |
| Lobular (bronchopneumonia | Bacteria |
| Confluent (lobar pneumonia) | Bacteria |
| With granules | Agents of botryomycosis (Staphylococcus aureus), actinomycosis (Actinomyces israelii) |
| Eosinophilic | Parasites |
| Foamy alveolar cast | Pneumocystis |
| Acute diffuse/localized alveolar damage | Virus; polymicrobial |
| Chronic pneumonia | |
| Fibroinflammatory | Bacteria |
| Organizing diffuse/localized alveolar damage | Virus |
| Eosinophilic | Parasite |
| Histiocytic | Mycobacteria |
| Interstitial pneumonia | |
| Perivascular lymphoid | Virus; atypical agents |
| Eosinophilic | Parasite |
| Granulomatous | Mycobacteria |
| Nodules | |
| Large | |
| Necrotizing | Fungi; mycobacteria |
| Granulomatous | Fungi; mycobacteria |
| Fibrocaseous | Fungi; mycobacteria |
| Calcified | Fungi; mycobacteria |
| Miliary | |
| Necrotizing | Viral; mycobacteria; fungi |
| Granulomatous | Fungi |
| Cavities and cysts | Fungi; mycobacteria |
| Intravascular/infarct | Fungi |
| Spindle cell pseudotumor | Mycobacteria |
| Minimal (“id”) reaction | Polymicrobial |
Useful Tissue Stains in Lung Infection
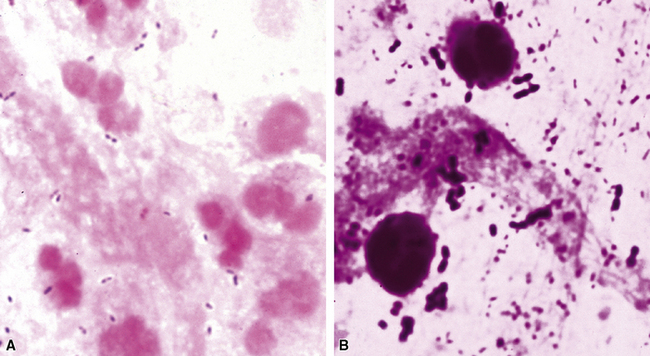
Many diagnostic pathologists have an aversion to the use of special stains for identifying organisms in tissue sections based on less than optimal specificity and sensitivity and the technical difficulty of performing some of these (especially silver impregnation methods, such as the Dieterle, Steiner, and Warthin-Starry stains). Nevertheless, several tissue section staining techniques are quite useful in detecting bacteria, mycobacteria, and fungi in tissue sections. A list of these is presented in Box 6-2. These stains should always be applied as part of an algorithmic strategy for acute lung injury, but especially in the immunocompromised patient.3 For example, when bacteria are being sought, some pathologists would prefer to begin with the tissue Gram stain (e.g., Brown and Hopps, Brown and Brenn) (Fig. 6-10), but silver impregnation techniques (e.g., Warthin-Starry) are actually more sensitive and a good starting point for approaching a suspected bacterial infection. By coating the bacteria with metallic silver, the bacterial silhouettes are enhanced (Fig. 6-11) and become more visible.3 Other stains (e.g., Giemsa) will sometimes detect bacteria that do not stain well with more conventional stains (Fig. 6-12). The Grocott methenamine silver (GMS) stain (Fig. 6-13) is the best stain for most fungi in tissue and also stains actinomycetes, Nocardia, Pneumocystis (cysts), free-living soil amebae, algal cells, the spores of certain microsporidia, and the cytoplasmic inclusions of cytomegalovirus (CMV).8
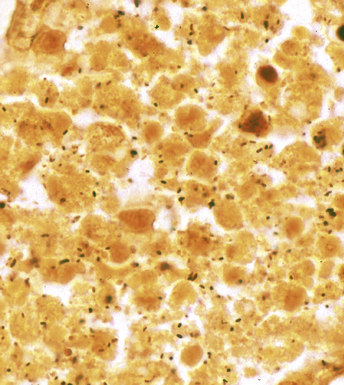
Figure 6-11 Black (silver-coated) bacilli (Legionella pneumophila) in alveolar exudate (Dieterle stain).
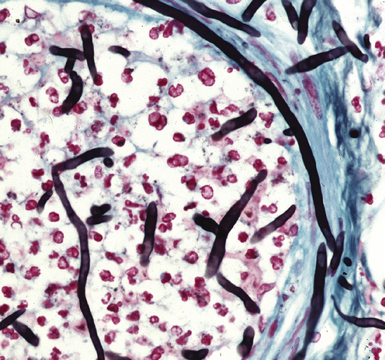
Figure 6-13 Angioinvasive Aspergillus species (Grocott methenamine silver stain).
(Courtesy of Dr. Francis Chandler, Augusta, GA.)
Most mycobacteria stain well with the Ziehl-Neelsen procedure (Fig. 6-14), but the auramine-rhodamine fluorescent procedure is superior in terms of sensitivity (Fig. 6-15). Nocardia organisms, Legionella micdadei, and Rhodococcus equi are weakly or partially acid-fast, and use of modified acid-fast stains such as in the Fite-Faraco technique is more satisfactory for identification of these organisms. Some mycobacterial species, such as M. avium complex (MAC), also are periodic acid/Schiff reagent (PAS)-positive, GMS-positive, and weakly gram-positive.
Finally, for completeness, it can be said that for identification of most protozoa and helminths, as well as viral inclusions, a good-quality hematoxylin and eosin (H&E)-stained section suffices; in fact, a well-prepared H&E section alone is diagnostic for many infectious diseases. This stain often can detect and even distinguish between bacterial cocci and bacilli when the burden of organisms is high (Fig. 6-16).
Immunologic and Molecular Techniques
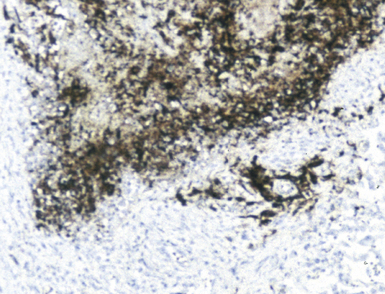
The application of ancillary studies, such as immunohistochemistry, in situ hybridization31 (Fig. 6-17), or nucleic acid amplification technology, can provide a specific etiologic diagnosis in certain cases. These techniques have the best chance for diagnosing infections caused by fastidious species that are difficult or impossible to culture from fresh samples, and also for situations in which only formalin-fixed, paraffin-embedded tissues are available. Immunohistochemical reagents for microbiological detection are becoming increasingly available and provide added power to determining specific diagnoses on formalin-fixed paraffin-embedded tissue32 (Fig. 6-18). Although these techniques provide the diagnostic equivalence of culture confirmation, they are not without limitations and diagnostic pitfalls. The PCR method first introduced in the 1980s has undergone a number of modifications. Non-PCR DNA amplification methods and methods based not on the amplification of the DNA target per se, but on amplification of the signal or probe have also been introduced.33 Among the more recently available technologies is the rapid-cycle “real-time” PCR assay, representing an especially powerful advance in that it is significantly more sensitive than culture. The adaptation of various amplification methods to real-time and multiplex formats enables laboratories to detect a wide range of respiratory pathogens. Furthermore, the transition from traditional and analyte-specific methods to more global technologies such as PCR arrays, liquid bead arrays, microarrays, and high-throughput DNA sequencing is under way. Over time, these methods will find a place in laboratories of all sizes, and dramatically impact the speed and accuracy of microbiologic testing practice for all types of microorganisms.34–37
Limiting Factors in Diagnosis
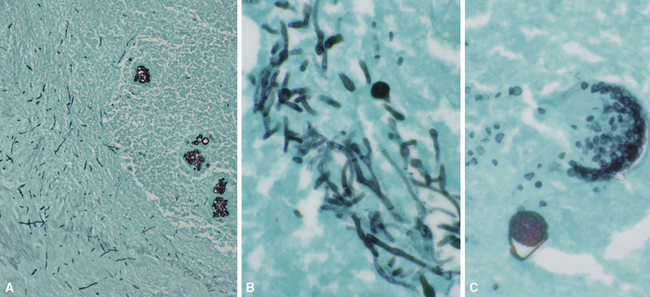
Needless to say, the diagnostic tools employed by both pathologists and microbiologists have their limitations, in terms of sensitivity and specificity.8 Some common tools are listed in Box 6-3. Culture alone cannot distinguish contamination from colonization, or in the case of viruses, asymptomatic shedding from true infection. Molecular tests also suffer from some of these problems; require specialized, often costly equipment; and are susceptible to false positive and false negative results.36 If a surgical biopsy is available, correlation of the histopathologic features can help assign an etiologic role to an agent recovered in culture. The host inflammatory pattern and morphologic features of an organism can be characteristic for certain types of infections, but often the organism’s morphology alone is not sufficient for a diagnosis at the genus or species level. Furthermore, the classic histopathologic findings for a given infection may be incomplete or lacking, making specific morphologic diagnosis possible for relatively few organisms. For example, the etiologic diagnosis is straightforward when large spherules with endospores characteristic of Coccidioides species are present, when small budding yeasts of Histoplasma capsulatum are seen, or yeasts with large mucoid capsules of Cryptococcus neoformans are identified. However, atypical forms of these organisms can be confusing.38 Similarly, hyphal morphology is helpful when it is characteristic of a specific genus or group, but the many look-alikes (Fig. 6-19) require separation by searching for subtle differences under high magnification (or oil immersion), or reliance on special techniques and culture.39
Box 6-3 Limitations of Diagnostic Tools
Morphology
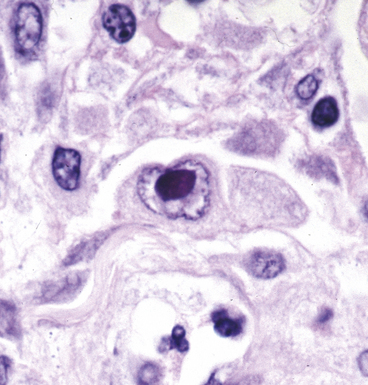
Certain viruses may have characteristic inclusions in tissue, but there are notable pitfalls. For example, eosinophilic intranuclear inclusions of adenovirus may resemble the early inclusions in herpes simplex virus or CMV, especially when the typical smudged cellular forms of adenovirus are absent. Also, simulators of viral cytopathic effect (CPE) can occur in a number of conditions and need to be recognized, such as macronucleoli, optically clear nuclei, and intranuclear cytoplasmic invaginations (Fig. 6-20).
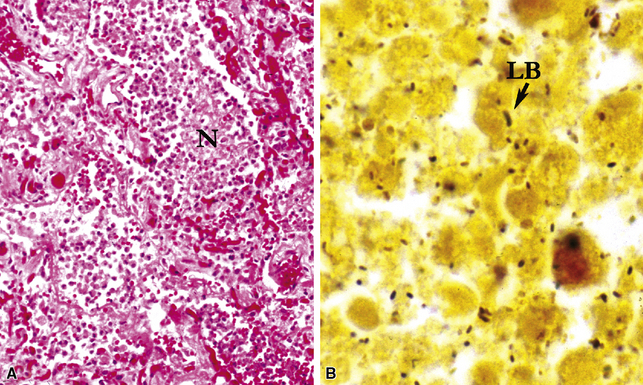
Pseudo-microbe artifacts also have been recognized on routine and special stains for identification of bacteria and fungi. Such potential artifacts include fragmented reticulin fibers, pigments, calcium deposits, Hamazaki-Wesenberg (yellow-brown) yeast-like bodies (Fig. 6-21), pollen grains, and even lymphoglandular bodies.40 For all of these reasons, the pathologist must maintain a high threshold for diagnosing organisms on morphologic grounds. If any question remains, it is best to repeat special stains liberally on deeper levels or in different tissue blocks.
The Role of Cytopathologic Examination in Diagnosis of Lung Infection
A wide variety of infectious diseases of the lung, including bacterial, mycobacterial, fungal, viral, and parasitic, can be diagnosed through exfoliative or fine-needle aspiration cytologic techniques.41–44 Fine-needle aspiration is an especially powerful tool, compared with exfoliative cytology study of respiratory secretions—sputum samples, bronchial washings, brushings, and bronchoalveolar lavage (BAL) fluid samples. The usefulness of exfoliative cytology examination often is limited owing to problems associated with distinguishing colonizing or oral contaminant organisms in the airways from true pathogens. Nonetheless, both diagnostic techniques are complementary and have been used in recent years to evaluate pneumonias and pulmonary nodules in both immunocompetent and immunocompromised patients.
Mass-like infiltrates are often the target of aspiration biopsy needles when suspicion or exclusion of an infectious process ranks high in the differential diagnosis. Besides the morphologic features of the microorganism, important cytologic clues to the diagnosis include the accompanying cellular response and the presence and character of any necrotic debris present, as outlined in Table 6-4. Although nonspecific, such features can suggest certain possibilities to the cytopathologist and assist the microbiology laboratory in triaging the specimen.45 To this end, the presence of a cytopathologist, microscope, and staining setup during the aspiration process can be useful. The cytopathologist can correlate the clinical setting, radiologic features, and clues from the gross character of the aspirate (color, consistency, odor, and so on), thereby assisting in narrowing the diagnostic possibilities and avoiding false-positive and false-negative diagnoses.46 Also, immediate evaluation of smears by rapid stain procedures allows the cytopathologist to either make or suggest a specific diagnosis, as with preparation and evaluation of a frozen section during intraoperative consultation. Smears can be prepared for special stains, needle rinses can be performed for culture and other ancillary studies, and additional aspirations may be encouraged for these purposes.47 Special stains for bacteria, mycobacteria, and fungi should be used whenever the character of the aspirate and the clinical setting (e.g., compromised immune status) indicate that such studies may be useful.
Table 6-4 Fine-Needle Aspiration (FNA) Patterns of Pulmonary Infectious Diseases
| Pattern | Possible Etiologic Agent(s) |
|---|---|
| Acute purulent inflammation/ abscess | Bacteria Fungi |
| Granuloma pattern (epithelioid cells with or without necrosis): Caseous/necrotizing Suppurative Epithelial Mixed | Mycobacteria Bacteria Parasite Fungi |
| Foamy alveolar cast pattern | Pneumocystis jiroveci |
| Histiocytic | Mycobacteria Bacteria Fungi |
| Chronic inflammation (lymphocyte and plasma cell) | Virus Other agent not otherwise specified |
| Null (“id”) reaction | Virus Any other |
Some interventionists prefer to provide only a needle core biopsy in lieu of an aspirate for a variety of reasons. These two techniques can be viewed as complementary; while needle core biopsies work well for neoplasms and many granulomas, the aspirate is often superior for diagnosing many types of infections, especially bacterial abscesses. Sometimes a rapid and specific etiologic diagnosis is possible at the bedside, based on the microscopic features of the organism itself. However, when the organism is not readily apparent or its features are inconclusive, the microbiology laboratory can be invaluable for its role in isolation and identification.47
Summary
The successful treatment of pulmonary infections depends on accurate identification of the pathogen involved. In turn, this requires collecting the best specimens, transporting them to the anatomic and microbiology sections of the laboratory under optimal conditions, and processing them with techniques appropriate for the spectrum of possible etiologic disorders. An interdisiplinary approach enhances this process. It is in the best interest of all parties involved that pathologists, clinicians, and microbiologists communicate frequently and recognize the strengths and weaknesses of their respective disciplines. Joint strategies can be developed for the approach to certain types of suspected infections, helping to foster the development of laboratory “foresight” in surgical colleagues and medical consultants. As methods of diagnosis, treatment, and antimicrobial prophylaxis change, the pathologist must remain vigilant to a changing spectrum of etiologic agents and tissue injury patterns. The pathologist capable of integrating clinical and imaging data with morphologic and microbiologic findings can construct a comprehensive report useful for patient managment. The microbiology laboratory can be instrumental in delivery of more effective and efficient patient management if the microbiologist can capture this information, in view of its optimal position for choosing the appropriate combination of diagnostic methods (morphologic, culture, immunologic, molecular) for a particular type of sample. An example of such an operational protocol is presented in Box 6-4.
Bacterial Pneumonias
The surgical pathologist rarely receives biopsy specimens from patients with community-acquired or nosocomial pneumonias. Most of these infections are suspected clinically by symptoms and physical and radiologic findings; some are confirmed immediately by Gram stains (or later by culture) performed on respiratory secretions in the microbiology laboratory. Serologic studies sometimes prove to be diagnostic. Even when conventional microbiologic approaches are applied, however, approximately 50% of bacterial pneumonias remain undiagnosed.48–50 Patients with mild disease often are not tested but simply treated empirically with antibiotic regimens following established guidelines. By contrast, patients with severe disease, whether immunocompromised or not, often become candidates for invasive procedures.
Etiologic Agents
Bacterial pneumonia may be classified according to various parameters including pathogenesis, epidemiology, anatomic pattern, clinical course, and organism type51 (Box 6-5). Using bacterial type as a starting point allows the pathologist to correlate anatomic and histopathologic patterns of lung injury with categories of etiologic agents.
The pyogenic bacteria most commonly associated with community-acquired pneumonias include S. pneumoniae, H. influenzae, and Moraxella catarrhalis.50 Other pathogens such as Legionella species, Chlamydia pneumoniae, and Mycoplasma pneumoniae (often referred to as the “atypical” group) are clinically important, but controversy exists with regard to the relative frequency of these organisms as etiologic agents. Although community-acquired pneumonia is considered to be fundamentally different in children and in adults, severe or complicated pneumonias in both of these age groups are of similar etiology.52 The enteric gram-negative bacilli cause relatively few community-acquired pneumonias, whereas they account for most of the nosocomial pneumonias, along with Pseudomonas species, Acinetobacter species, S. aureus, and anaerobes.53,54Most nosocomial pneumonias result from aspiration of these bacterial species that colonize the oropharynx of hospitalized patients, and they are often polymicrobial. Any of the bacterial organisms listed (including mixtures with fungi and viruses) can cause pneumonia in immunocompromised patients.15,55 Ventilator-associated pneumonia is a special subset of nosocomial pneumonia and an important cause of morbidity and mortality in the intensive care unit.56–58 The bacterial etiology in this setting is quite diverse and dependent on such factors as patient characteristics, underlying lung disease, and geographic location.59 Most recently, an increase in skin and soft tissue staphylococcal infections due to methicillin-resistant strains has led to the recognition of these organisms as an important cause of both community-acquired and nosocomial pneumonia with attendant morbidity and mortality.60 In rare nosocomial pneumonias, a number of unusual organisms, such as Salmonella, Rhodococcus, and Leptospira species, may be the etiologic agent.61,62
The atypical pneumonia agents are those that do not commonly produce lobar consolidation. Although this potentially implicates a wide variety of bacterial, viral, and protozoal pathogens, a selective list by convention includes Mycoplasma pneumoniae, Legionella species, and C. pneumoniae as the three dominant nonzoonotic pathogens, and Coxiella burnetii (the agent of Q fever), Chlamydia psittaci (causing psittacosis in people), and F. tularensis (causing tularemia) as the three more common zoonotic pathogens.63,64
The filamentous/granule group refers to those bacteria that form long, thin, branching filaments in tissues, such as Actinomyces (anaerobic actinomycetes) or Nocardia (aerobic actinomycetes).65 Botryomycosis is caused by nonfilamentous bacteria, especially Staphylococcus aureus, or gram-negative bacilli, such as P. aeruginosa and E. coli, which form organized aggregates referred to as grains or granules.66
Histopathology
Bacterial lung injury patterns will vary in accordance with the virulence of the organism and the host response. These patterns are further modulated by therapeutic or immunologic factors. Although some of the patterns presented in Box 6-6 are characteristic, none are diagnostic. Overlap and mixed patterns occur.
Acute Exudative Pneumonia
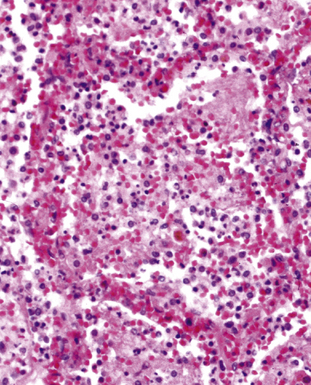
Acute exudative pneumonia most often is caused by pyogenic bacteria, such as streptococci, which typically produce a neutrophil-rich intra-alveolar exudate (i.e., alveolar filling) with variable amounts of fibrin and red cells. Pathologists recognize this constellation of findings as acute lobular pneumonia (Fig. 6-22), which usually correlates with patchy segmental infiltrates on the chest film (consolidation pattern on HRCT).29,67–69
With increasing organism virulence and disease severity, lobular exudates may become confluent (i.e., lobar pneumonia). In milder cases, the disease may be limited to the airways (bronchitis/bronchiolitis) with a mixed cellular infiltrate of mononuclear cells and neutrophils (Fig. 6-23). One very common manifestation of such airway-limited infection has been designated as “acute exacerbation of chronic obstructive pulmonary disease” (COPD). A majority of these exacerbations are caused by particular bacteria, specifically H. influenzae, S. pneumoniae, and M. catarrhalis, with approximately one third resulting from viral airway infections, typically resulting from rhinovirus, respiratory syncytial virus (RSV), and human metapneumovirus.70
Nodular/Necrotizing Lesions
Nodular inflammatory infiltrates with or without necrotizing features (Fig. 6-24) are characteristic of infection by certain species, such as Rhodococcus equi (Fig. 6-25).71 Necrotizing pneumonias also may be produced by pyogenic bacteria such as Staphylococcus aureus, Streptococcus pyogenes, and the gram-negative bacilli—Klebsiella, Acinetobacter, Pseudomonas, and Burkholderia species.
Miliary Lesions
A subset of the nodular histopathologic pattern, miliary infection (Fig. 6-26), strongly implies pneumonia secondary to hematogenous spread of bacteria (septicemia). This pattern of infection can be seen with other organisms, such as Nocardia and the anaerobic actionomycetes. In these settings, histopathologic examination may show a hybrid reaction with both nodular disease and alveolar filling.
Aspiration Pneumonia and Lung Abscess
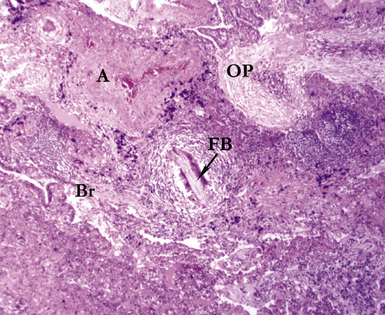
Several pulmonary aspiration scenarios are recognized, including those caused by chemical pneumonitis (so-called Mendelsson syndrome), airway obstruction, exogenous lipoid pneumonia, chronic interstitial fibrosis, diffuse bronchiolar disease, bacterial pneumonia, and lung abscess.72,73 Aspiration pneumonia refers specifically to aspiration of bacteria in oropharyngeal secretions and is classically a polymicrobial aerobic/anaerobic bacterial infection, with the bacterial species depending on whether the aspiration event occurs in the community or hospital setting. Recognition of food particles (so-called pulses) is key to the diagnosis. These may or may not be invested by giant cells but usually are found in purulent exudate or granulomatous foci. In the organizing phase of the pneumonia, food particles may be found within polyps of organizing pneumonia in the alveolar ducts and alveoli. Lobular pneumonia, lipoid pneumonia, organizing pneumonia, and bronchiolitis, alone or in combination, also may be seen.69,74 The pathogens in lung abscess (Fig. 6-27) usually encompass a polymicrobic mixture of aerobic and anaerobic bacteria,75 and formation of such abscesses most often is secondary to aspiration (Fig. 6-28). Infections due to Actinomyces species (Fig. 6-29) and Nocardia species also may manifest this pattern, as can those due to certain pyogenic bacteria, such as Staphylococcus aureus and the other organisms listed previously for necrotizing pneumonias. Granulomatous inflammation with foreign bodies may be present if aspiration is the cause (Fig. 6-30).
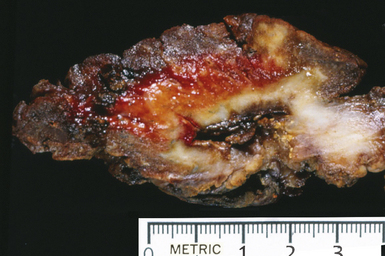
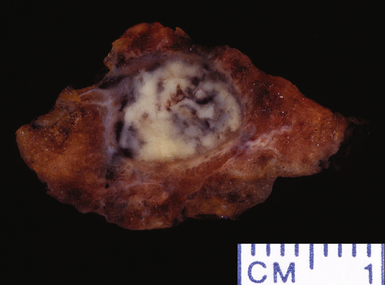
Figure 6-27 Lung abscess showing gross evidence of chronicity with fibrosis in surrounding parenchyma.
Chronic Bacterial Pneumonias
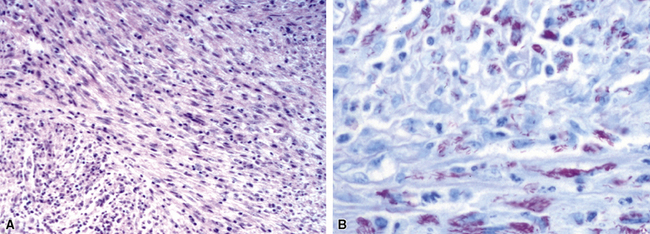
Chronic bacterial infections (Fig. 6-31) that are slow to resolve as a result of inappropriate initial therapy, involvement with certain microbial species, a noninfectious comorbid process, or an inadequate host response can produce a nonspecific fibroinflammatory pattern, with lymphoplasmacytic infiltrates, macrophages, or organization with polyps of immature fibroblasts in alveolar ducts and alveolar spaces.76–79 If not resorbed, polyps of air space organization may become polyps of intra-alveolar fibrosis, which sometimes ossify (dendriform ossification). Such scarring in chronic pneumonia often is associated with localized interlobular septal and pleural thickening (Fig. 6-32), producing a “jigsaw puzzle” pattern of scarring best seen at scanning magnification.
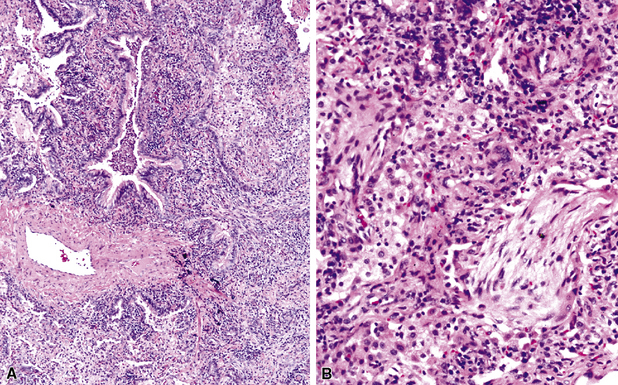
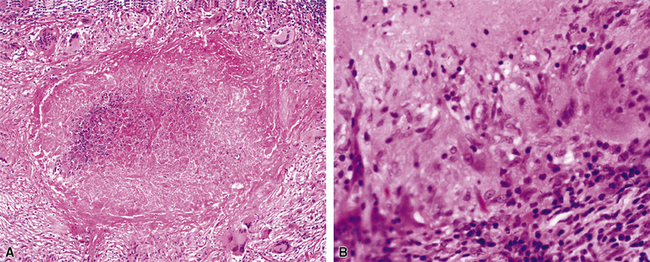
Figure 6-31 Chronic pneumonia.
A, lymphoplasmacytic infiltrate. B, fascicles of fibroblasts in alveolar ducts and spaces.
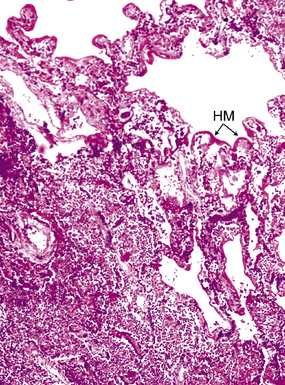
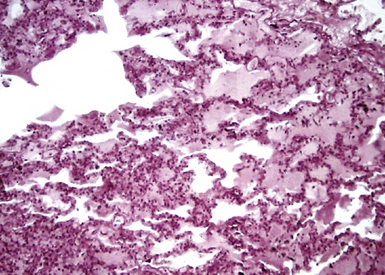
Diffuse alveolar damage is the histopathologic correlate of the acute repiratory distress syndrome (ARDS), and today, lung infection is the leading cause of diffuse alveolar damage and ARDS in the United States.80 Diffuse alveolar damage may coexist with any of the necroinflammatory patterns described earlier. The initial exudative phase of this process is accompanied by hyaline membranes (Fig. 6-33); the later organizing phase is attended by air space and interstitial fibroplasia. In clinical practice, diffuse alveolar damage accompanied by tissue necrosis is nearly always a manifestation of lung infection.
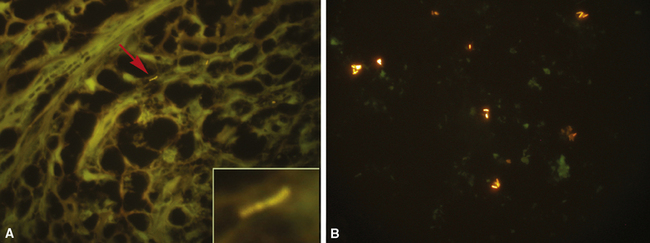
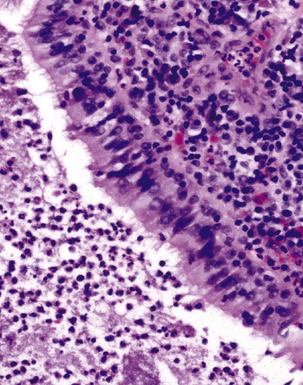
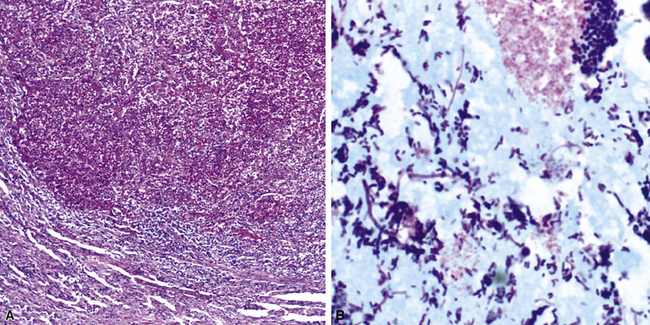
The atypical pneumonias include the well-described cases due to Legionella species and the less well-described cases caused by other organisims comprising the atypical group. Legionella infection typically results in an intensely neutrophilic acute fibrinopurulent lobular pneumonia64,67 (Fig. 6-34A). Legionella bacilli often can be identified in silver impregnation-stained sections (see Fig. 6-34B) or recovered in culture, but newer diagnostic methods, such as real-time PCR and in situ hybridization (Fig. 6-35) also can be applied when standard approaches fail.81 The histopathologic patterns associated with the other members of the atypical group (i.e., Chlamydia, Mycoplasma) are not well characterized, mainly because investigation of these pneumonias rarely includes biopsy. The few well-documented cases of Mycoplasma, Chlamydia, and Coxiella infections that have been described in the literature resemble viral bronchitis or bronchiolitis, with mixed inflammatory infiltrates in airway walls and in the adjacent interstitium82,83 (Fig. 6-36). Relative sparing of the peribronchiolar alveolar spaces has been described, although patchy organized fibrinous exudates are seen in some cases, and complications may superimpose additional findings.
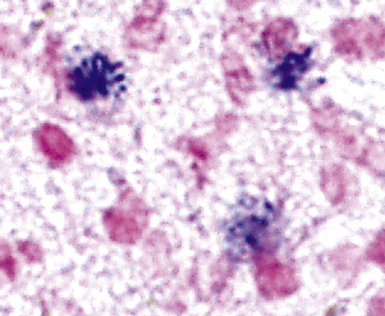
Figure 6-35 Legionnaire’s disease.
Detection of organisms by in situ DNA hybridization.
(Courtesy of R. V. Lloyd, MD, Rochester, MN.)
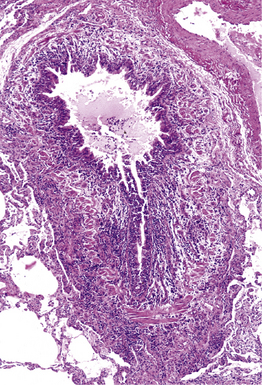
Figure 6-36 Mycoplasma pneumonia.
Bronchiolitis with patchy infiltrates in peribronchial interstitium.
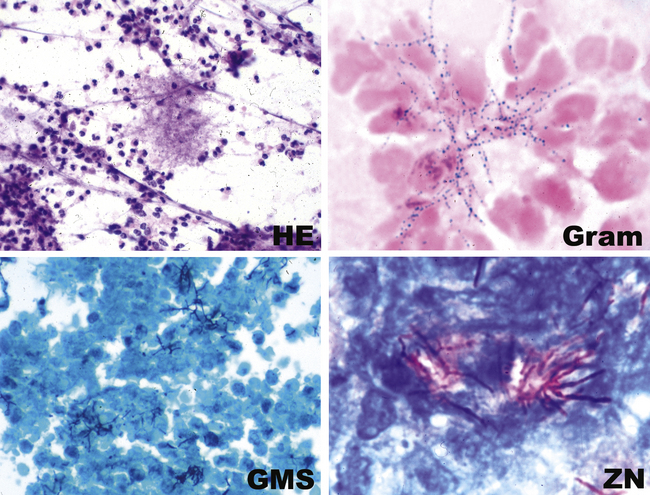
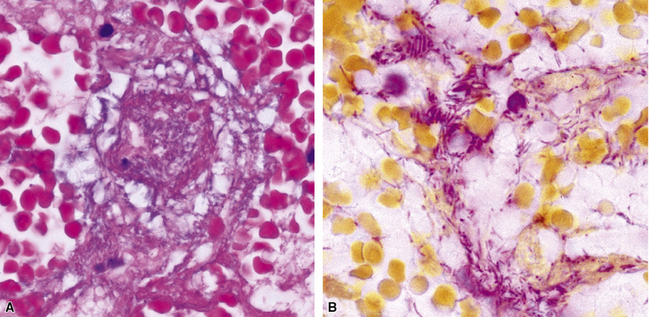
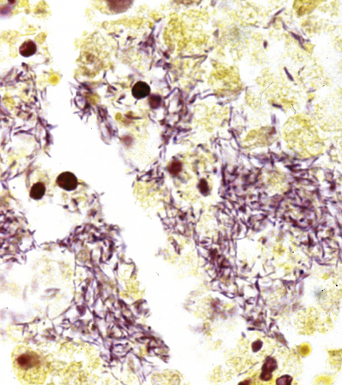
The grains and granules formed by the actinomycetes and bacteria of botryomycosis may have a uniform tinctorial hue on routine hematoxylin and eosin (H&E)–stained sections, but sometimes these bacterial aggregations display a distinctive body with a hematoxylinophilic core and an outer investment of eosinophilic material; formation of this array is referred to as the Splendore-Hoeppli phenomenon (Fig. 6-37). Actinomyces species tend to form similar-appearing granules, and both they and the bacteria of botryomycosis typically are found in the midst of purulent exudates.65,84–86 The Nocardia species may aggregate in colonies simulating granules, but with a much looser texture (Fig. 6-38) and more monochromatic tinctorial properties.87 Rarely, these colonies may be identical in appearance to the grains or granules of botryomycosis or actinomycosis in H&E sections.
Bacterial Agents of Bioterrorism
The potential for use of microbial pathogens as agents of bioterrorism requires that clinicians be alert to this possibility when community-acquired pneumonias are found to be caused by these agents. In turn, pathologists must become familiar with the histopathologic features these agents can produce.88 Respiratory disease caused by the inhalation of Bacillus anthracis, Yersinia pestis, and Franciscella tularensis is especially pertinent in this context and is discussed next.
Bacillus anthracis
In 1877, Robert Koch’s conclusive demonstration that B. anthracis was the etiologic agent of anthrax revolutionized medicine by linking microbial cause and effect.7 Set against its historical importance to medicine, the recent use of anthrax as a bioterrorism agent represents a sad contrast. Inhalational anthrax causes a severe hemorrhagic mediastinitis.89–93 This pathologic process, in combination with the toxemia (B. anthracis produces an exotoxin with three potent components—protective antigen, lethal factor, and edema factor) from the ensuing massive bacteremia, severely compromises pulmonary function, leading to death in 40% or more of the cases. Pleural effusion may be present, but pneumonia generally is minor and secondary. In those patients in whom pulmonary parenchymal changes are found, the alveolar spaces contain a serosanguineous fluid with minimal fibrin deposits and some mononuclear cells, but few if any neutrophils.92 Large gram-positive bacilli (some may appear partially gram-negative) without spores, pervade the alveolar septal vessels, with a few in the alveolar spaces. This distribution suggests hematogenous rather than airway acquisition. Hemorrhagic mediastinitis in a previously healthy adult is essentially pathognomonic for inhalational anthrax. The lymph node parenchyma generally is teeming with intact and fragmented gram-positive bacilli, which can be identified as B. anthracis by immunohistochemical studies.91,92 Cultures of blood and pleural fluid, if available, are likely to yield the earliest positive diagnostic results.93 Sputum studies are much less useful in this regard.
Yersinia pestis
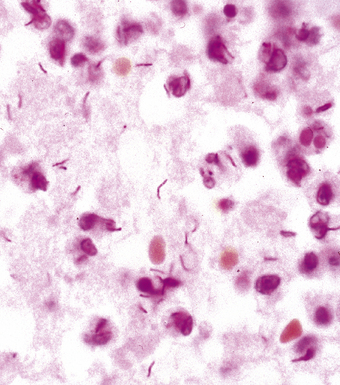
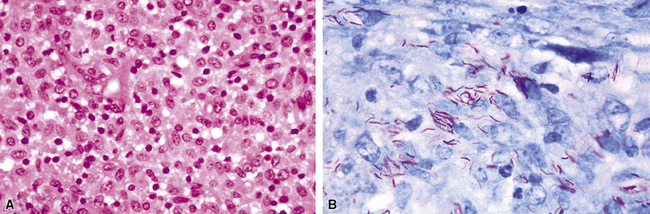
Primary pneumonic plague follows inhalation of Y. pestis bacilli in a potential bioterrorism scenario.94 The infection begins as bronchiolitis and alveolitis that progress to a lobular and eventual lobar consolidation.95 The histopathologic features evolve over time, beginning with serosanguineous intra-alveolar fluid accumulation with variable fibrin deposits (Fig. 6-39), progressing through a fibrinopurulent phase, and culminating in a necrotizing lesion.96 The presence of myriad bacilli in the intra-alveolar exudates, with significantly fewer organisms in the interstitium (a characteristic of primary pneumonia), is one of several pulmonary and extrapulmonary features used to distinguish primary from secondary pneumonic plague.97 These bacilli may be obvious in H&E-stained sections (Fig. 6-40) but generally are better visualized with Giemsa rather than Gram stain. Immunohistochemical staining provides a rapid and specific diagnosis.95 Unlike with inhalational anthrax, sputum Gram stain and culture are useful tests that are likely to yield a positive result at clinical presentation. Also, because sepsis is an integral component of the pneumonia, it is important to collect blood culture specimens.
Francisella tularensis
Inhalation of F. tularensis bacilli, following a bioterrorism aerosol release, generally is expected to result in a slowly progressing pneumonia, with a lower case-fatality rate than with either inhalational anthrax or plague.97 Initially, a hemorrhagic and ulcerative bronchiolitis is followed by a fibrinous lobular pneumonia with many macrophages but relatively few neutrophils (Fig. 6-41). Necrosis then supervenes and evolves into a granulomatous reaction. The small, gram-negative coccobacillary organisms are difficult to identify in a tissue Gram stain, and the use of silvering techniques (e.g., Steiner, Dieterle, Warthin-Starry) is required to enhance their silhouette.98 Specific fluorescent antibody testing for formalin-fixed tissue and immunohistochemical studies also are available through public health laboratories. In the microbiology laboratory, Gram stain and culture of respiratory secretions are useful for diagnosis, but blood culture results are not often positive. Antigen detection and molecular techniques, such as PCR amplification, can be used to identify F. tularensis. Serologic tests are available but probably would not provide timely information in an outbreak situation.97
Cytopathology
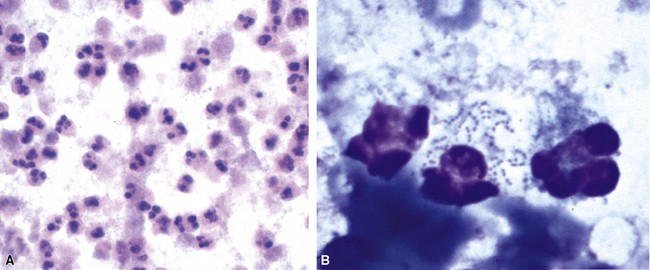
The stereotypic cellular response to pyogenic bacteria is acute inflammation, characterized by variable numbers of neutrophils. Bacteria may be visualized in various stained preparations made from respiratory tract secretions and washings using the Papanicolaou and Diff-Quik methods.43 The clinical significance is rather limited in these specimens owing to the potential contamination by oral flora and the problem of distinguishing colonization from infection. However, when the upper respiratory tract can be bypassed, by means of either transtracheal or transthoracic needle aspiration, the presence of bacteria becomes much more significant, especially when sheets of neutrophils or necroinflammatory debris are present (Fig. 6-42A), as would be the case with a typical lobar or lobular consolidation, lung abscess, or other complex pneumonia.49,86,99,100 In this context, transthoracic needle aspiration can establish the etiologic diagnosis of community-ascquired and nosocomial pneumonias in both children and adults when coupled with modern microbiologic methods.47,54,101,102 Proponents consider it an underutilized technique whose potential benefits, in experienced hands, outweigh the modest associated risks.
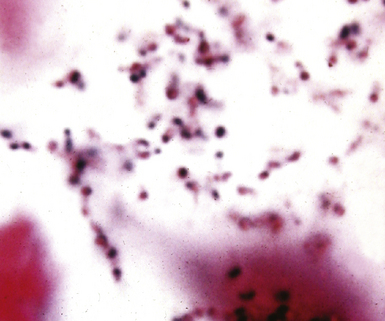
Many types of bacilli and cocci can be seen within and around neutrophils on Diff-Quik–stained smears (see Fig. 6-42B). A smear also can be prepared for Gram stain and the aspirate needle rinsed in nonbacteriostatic sterile saline or nutrient broths for culture. The size (length and width) and shape of organisms and the Gram reaction allow rough categorization of organisms into groups such as enteric-type bacilli, pseudomonads, fusiform anaerobic-type bacilli, tiny coccobacillary types suggestive of the Haemophilus–Bacteroides group (Fig. 6-43), or gram-positive cocci.103 Branching filamentous forms suggest actinomycetes or Nocardia organisms (Fig. 6-44), with the latter distinguished by being partially acid-fast.104,105 Extreme care must be exercised in the staining laboratory to prevent contamination of staining solutions, because this can be a cause of false-positive results.
Microbiology
Microbiology techniques in current use for the laboratory diagnosis of bacterial pneumonia are summarized in Box 6-7.106–108 The traditional morphologic and functional approach to microbiologic diagnosis is gradually shifting to molecular methods, but their routine application continues to be a hope for the near future.
The workup of respiratory secretions, such as sputum, in the microbiology laboratory may or may not be indicated, based on the clinical and immunologic status of the patient. Certainly, the value of this workup for community-acquired pneumonias has been questioned for some time, and the guidelines from two specialty societies—the American Thoracic Society and the Infectious Disease Society of America—differ in this regard.109–111 Nevertheless, when a carefully collected specimen reveals one or two predominant bacterial morphotypes on a well-prepared Gram stain (Fig. 6-45), especially in the presence of neutrophils and few or no squamous cells, a presumptive diagnosis can be offered and correlated with whatever grows on culture plates.112,113 A mixed bacterial population usually is considered nondiagnostic, especially in the absence of inflammation or the presence of many benign oral squamous cells. By contrast, pneumonia in the hospitalized or immunocompromised patient requires an aggressive strategy to collect a good sputum sample for Gram stain and culture. If this attempt is unsatisfactory or the findings are nondiagnostic, then use of invasive techniques beginning with fiberoptic bronchocopy and BAL with protected catheters should be considered.56,58,114 Anaerobic pulmonary infections, typically in the form of a lung abscess, also can be approached in this way or with transthoracic needle aspiration.75
In those cases in which bacteria are visible on H&E-stained sections, the Gram stain is especially helpful in confirming a presumptive etiology. For example, pairs and chains of gram-positive cocci in a necroinflammatory background suggest a streptococcal pneumonia, whereas numerous slender gram-negative bacilli investing and infiltrating blood vessels are characteristic of a Pseudomonas pneumonia (Fig. 6-46). Other types of gram-negative pneumonias (Fig. 6-47) also can be confirmed with well-prepared Gram stains.77 In the case of an abscess, a mixture of gram-positive cocci and gram-negative bacilli in tissue (illustrated earlier in Fig. 6-28) is a useful finding that is helpful in supporting a diagnosis of an anaerobic infection.
When organisms are sparse, other stains such as Giemsa or silver impregnation may highlight the organisms in the exudates (Fig. 6-48). The Gram stain also is useful for evaluating infections with granules and allows differentiation of the agents of botryomycosis (the gram-positive cocci or gram-negative bacilli) from the filamentous Actinomyces organisms (Fig. 6-49).
Staining with methenamine silver is the best procedure for detecting Nocardia organisms. The modified Ziehl-Neelsen stain allows for differentiation of Nocardia (positive) from the anaerobic Actinomyces (negative).105
Commercially available immunohistochemical reagents exist for relatively few bacterial species. Immunohistochemistry testing for the potential bioterrorist agents discussed in this chapter is available through the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia. It is expected that commercial reagents will become increasingly available for the common etiologic agents in the near future.32
Culture media that will allow recovery of common bacterial species causing pneumonia from various types of respiratory samples (secretions, washings, brushings, aspirates, and tissues) include sheep blood agar, chocolate agar, and McConkey agar. These media also will support growth of B. anthracis and Y. pestis. Buffered charcoal yeast extract (BCYE) agar is the primary medium for Legionella species. Because Legionella organisms survive poorly in respiratory secretions, rapid transport and immediate plating is essential for recovery. BYE also is a good “all-purpose” medium for growing other fastidious species including F. tularensis. However, F. tularensis grows best in cysteine-enriched media.115
The actinomycetes are best isolated from invasive specimens such as needle aspirates and transbronchial and lung biopsy specimens. The laboratory should be alerted to search for these agents because special consideration must be given to culture setup and incubation conditions.85 The actinomycetes responsible for actinomycosis require anaerobic media and atmosphere as well as prolonged incubation. Nocardia, an aerobic actinomycete, grows well on most nonselective media but requires extended incubation. Determination of colonial morphology, Gram and acid-fast stains, and a few biochemical tests generally suffice to identify these organisms at the genus level. However, genotype rather than phenotype characteristics are required to identify newly emergent species.116
In general, the laboratory diagnosis of pneumonia caused by most of the atypical agents is difficult because systems are not routinely available or are costly, cumbersome, or unsafe. For the atypical agents (Mycoplasma, Chlamydia, and Coxiella species), serologic testing has been the method of choice for diagnosis.63,117 Classic cold agglutinin and complement fixation tests for these agents have largely been replaced by enzyme immunoassay and microimmunofluorescence testing.83,118,119 Serologic methods also are useful for diagnosis of tularemia because of the difficulty in culturing the fastidious bacterium.
Legionella pneumonia is a common form of severe pneumonia not readily diagnosed for a number of reasons, including the organism’s fastidiousness.120 In the microbiology laboratory, the direct fluorescent antibody test and culture on buffered BCYE agar have been the mainstays of diagnosis. Culture is considered the diagnostic gold standard but is only 60% sensitive. Serologic testing is available for most of the L. pneumophila serotypes, which account for 90% of the pneumonia cases; however, the need to collect paired sera weeks apart limits its usefulness in the acutely ill patient. Antigen detection in urine has become commercially available for both L. pnemophila and S. pneumoniae, and because the need to collect acute and convalesent sera is obviated, it has become a frequently used diagnostic test.120,121 Its advantage lies in its potential to effect early treatment decisions through rapid diagnosis. Its disadvantage lies in the fact that it identifies only patients infected with L. pneumophila serogroup 1 (LP1), the most prevalent species and serotype, but none of the non-LP1 serotypes, or cases due to other Legionella species.122–124
The use of molecular diagnostic tools (in situ hybridization and nucleic acid amplification by PCR or other methods) to detect these agents has been reported.81,124,125 Real-time PCR assay appears promising as a sensitive, specific, and rapid diagnostic technique that is likely to find routine clinical application. It provides a platform for the simultaneous amplification and detection of target DNA in a single tube through use of one of several types of fluorescence resonance transfer (FRET) fluorescent probe quencher techniques or melting curve analysis. Furthermore, it obviates the concern for amplicon contamination in the laboratory.126 The development of a multiplex assay, to detect multiple agents in a single reaction, would seem to be an ideal pursuit for the laboratory diagnosis of the most common community-acquired pneumonias including those due to the atypical pneumonia agents.35,127,128
Differential Diagnosis
The key morphologic and microbiologic features of the bacterial pneumonias are summarized in Table 6-5. The presence of purulent exudates or significant numbers of neutrophils in biopsy or cytologic samples should always trigger a search for bacterial infection. Of note, however, because lung biopsies usually are performed late in the clinical course with respect to an evolving infiltrate, after many procedures have been performed and bacterial infections have been excluded or treated with antibiotics, neutrophilic exudates may not signify bacterial infection unless accompanied by necrosis, as in an abscess. Instead, consideration should be given to one of several noninfectious acute inflammatory diseases, with an immunologic basis, that can mimic bacterial infection. Some of these include Wegener granulomatosis, Goodpasture syndrome, systemic lupus erythematosus, and microscopic polyangiitis, all conditions that can produce acute inflammation predominantly involving alveolar septal blood vessels (“capillaritis”). On occasion, capillaritis can result in air space accumulation of neutrophils, further raising concern for bronchopneumonia. Centrally necrotic or cavitary neoplasms of various types may mimic abscesses grossly and microscopically, and exceptionally well-differentiated adenocarcinomas containing glands filled with detritus may mimic inflammatory and bacterial diseases. Suppurative granulomas can have a bacterial, mycobacterial, or fungal etiology. Even the miliary necroinflammatory lesion typical of bacterial infection can be produced by viruses, some fungi, and even protozoa (e.g., Toxoplasma gondii).
Table 6-5 Bacterial Pneumonias: Summary of Pathologic Findings
| Assessment Component | Findings |
|---|---|
| Pyogenic Bacteria | |
| Surgical pathology | Acute purulent inflammation with/without necrosis; organization; diffuse alveolar damage may be present |
| Cytopathology | Acute inflammation with/without visible bacteria on Diff-Quik–stained smear |
| Microbiology | Gram stain reactivity and morphology (visual detection requires heavy bacterial burden: 106 organisms/gram of tissue) Culture-sterile lung tissue on standard nonselective and selective media (blood, chocolate, MacConkey agars); anaerobic broth and agars for abscesses Urinary antigen for Streptococcus pneumoniae |
| Atypical Pneumonia Agents | |
| Surgical pathology | Legionella pneumonia: fibrinopurulent with bacilli visible in silver-stained (Dieterle; Warthin-Starry) sections DAD often present Chlamydia and Mycoplasma infection: polymorphous bronchiolar and interstitial infiltrate |
| Cytopathology | Acute inflammation with bacilli stained with silver or by immunofluorescence (Legionella pneumonia) |
| Microbiology | DFA for L. pneumophila serotypes Culture on selective (BCYE) agar for Legionella; urinary antigen for Legionella Serologic testing and/or PCR assay for Mycoplasma and Chlamydia |
| Filamentous-Granule Group | |
| Surgical pathology | Granules or loose filamentous aggregates in purulent exudate with abscess formation and poorly formed granuloma in some cases |
| Cytopathology | Filamentous tangles or aggregates or granules with neutrophils and/or necroinflammatory background |
| Microbiology | Gram-positive branching filaments: Nocardia (aerobic actinomycete) and Actinomyces (anaerobic actinomycete) Nocardia partially acid-fast and GMS-positive Gram-positive cocci or gram-negative bacilli (botryomycosis) Culture on standard nonselective media and selective (BCYE) media; anaerobic culture broths and media for Actinomyces |
BCYE, buffered charcoal yeast extract; DAD, diffuse alveolar damage; DFA, direct fluorescence assay; GMS, Grocott methenamine silver.
Mycobacterial Infections
The surgical pathologist tends to encounter mycobacterial infections in lung biopsies when standard clinical diagnostic approaches to pulmonary infiltrates are unsuccessful and the lesions persist or progress. Tuberculosis is but one of several different types of lung infection that can manifest clinically as community-acquired pneumonia, resulting in delay until an invasive procedure such as transbronchial biopsy, transthoracic needle biopsy, or surgical lung biopsy is performed, often a “last resort” effort.129,130 In recent years, delays in diagnosis of mycobacterial infection have markedly decreased, thanks in part to recommendations from the CDC for improving laboratory turnaround time and to the response of the diagnostics industry with better methods and technology. In fact, however, because direct acid-fast bacillary smears of respiratory specimens yield negative findings in at least one half of the cases,131 and because many mycobacterial species are fastidious and slow-growing, the biopsy results may be the first suggestion of a mycobacterial infection. The biopsy findings also can define the organism’s relationship to a histopathologic lesion, or host response. This is important in evaluating the significance of a culture result, because although an isolate of M. tuberculosis is always taken seriously, obtaining a single isolate of a nontuberculous mycobacterium from the respiratory tract does not necessarily implicate the organism as the cause of disease.132
Etiologic Agents
Mycobacterium tuberculosis
M. tuberculosis is the most virulent mycobacterial species and an unequivocal pathogen that is responsible for more deaths worldwide than any single microbe. This organism is the etiologic agent of tuberculosis worldwide in its various forms, which are listed in Box 6-8.
Box 6-8 Classification of Tuberculosis
Data from Allen E. Tuberculosis and other mycobacterial infections of the lung. In: Churg AM, Thurlbeck WM, eds. Pathology of the Lung, 2nd ed. New York: Thieme; 1995:233, Table 13-1.
Primary tuberculosis occurs in patients without previous exposure or loss of acquired immunity. Progressive primary tuberculosis occurs in patients with inadequate acquired immunity, that is, impaired cellular immunity. Post-primary tuberculosis, also referred to as secondary or reinfection-reactivation tuberculosis, occurs in patients with previous immunity to the organism and accounts for most clinical cases of tuberculosis.133,134 Many clinical experts consider that most cases of active tuberculosis in adults with normal immunity arise from reactivation of latent infection (post-primary tuberculosis), whereas reinfection with a new strain derived from the environment (primary or post-primary tuberculosis) can occur in the immunocompromised patient. More recently, DNA fingerprinting methods (genotyping) have challenged this dogma, however, by showing that exogenous reinfection accounts for a significant percentage of cases in some areas of the world.135 Miliary tuberculosis and extrapulmonary disease can occur with any of these forms.133,136
Primary tuberculosis usually is a mild illness that often is not clinically recognized. Of note, however, the bacillemia that occurs during its development can seed extrapulmonary organs and set the stage for subsequent reactivation. Approximately 5% of patients pass through latency to post-primary disease within 2 years of primary infection, and another 5% do so later in their lives.137
Non-Tuberculous Mycobacteria
Recognized NTM species number more than 125, many of which were identified during the past decade.138,139 However, relatively few cause pulmonary disease.132,140–142 These organisms are acquired from the environment, where they are ubiquitous. In contrast with M. tuberculosis, the NTM are not spread from person to person. In most instances, patients in whom NTM infection develops have chronic lung disease and other risk factors, such as AIDS, alcoholism, or diabetes. Reports of NTM infections in non-immunocompromised patients are increasing.17,143 MAC and then Mycobacterium kansasii are the most frequent isolates in all settings. Among a growing number of species causing lung disease are Mycobacterium abscessus, Mycobacterium fortuitum, Mycobacterium szulgai, Mycobacterium simiae, Mycobacterium xenopi, Mycobacterium malmoense, Mycobacterium celatum, Mycobacterium asiaticum, and Mycobacterium shimodii. These latter species manifest marked geographic variability with respect to prevalence and severity. Of note, however, since 1985, more MAC isolates than M. tuberculosis have been reported in the United States.132
Histopathology
The histopathologic patterns produced by mycobacteria are listed in Box 6-9. The radiologic, gross, and microscopic patterns of mycobacterial disease reflect the virulence of the various mycobacterial species, as well as the patient’s prior exposure and immune status.144–146
Box 6-9 Histopathologic Patterns in Mycobacterial Lung Injury
Primary Tuberculosis
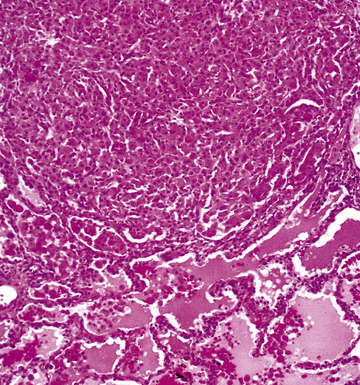
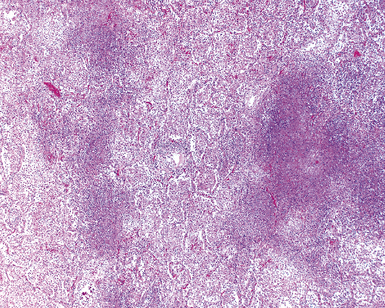
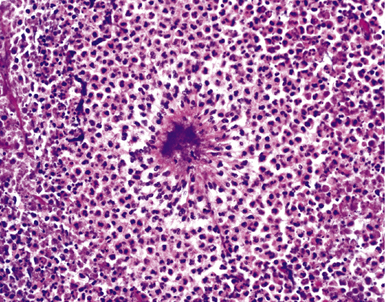
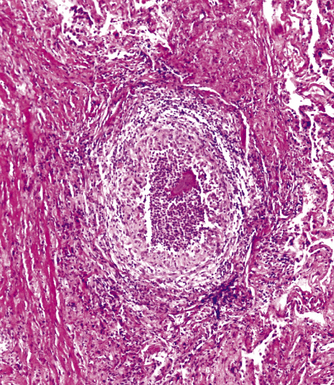
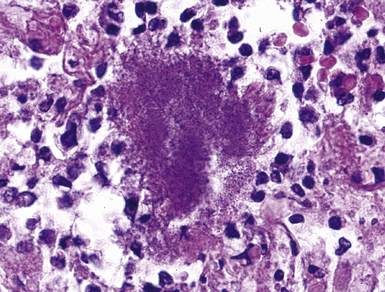
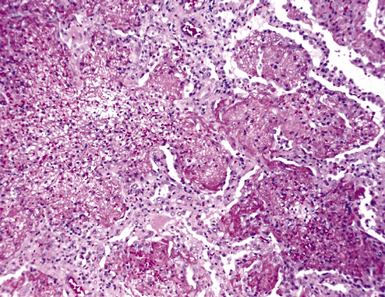
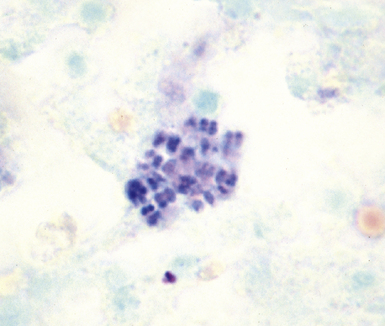
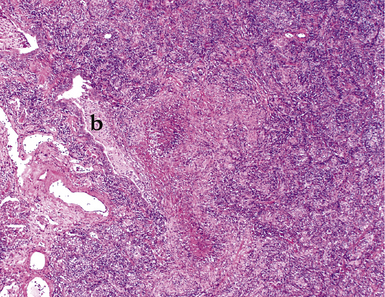
Mycobacterium tuberculosis occurs typically in the best-aerated lung regions (anterior segments of the upper lobes, lingua and middle lobe, or basal segments of lower lobes.145 The disease passes through progressive phases of exudation, recruitment of macrophages and T lymphocytes, and granuloma formation followed by repair with granulation tissue, fibrosis, and mineralization.134,147 Macrophage-laden bacilli also travel to the hilar lymph nodes, where the phases are repeated. This combination of events produces the classic Ghon complex, consisting of a peripheral 1- to 2-cm lung nodule (Fig. 6-50) and an enlarged, sometimes calcified hilar lymph node. In both locations, the histopathologic hallmark is a necrotizing granuloma (Fig. 6-51) composed of epithelioid cells with variable numbers of Langhans giant cells, a peripheral investment of lymphocytes, and a central zone of caseation necrosis, a form of necrosis attributed to apoptosis.133,148 A spectrum of lesions may be seen, from the tuberculoid “hard” granuloma without necrosis and rare organisms, to the multibacillary necrotic lesion with scant epithelioid cells.149 In a minority of patients the lesions enlarge and progress as a result of increased necrosis or liquifaction.
The complications of tuberculosis are listed in Box 6-10 and illustrated in Figure 6-52. Other complications may include extension into blood vessels with miliary (Fig. 6-53) or systemic dissemination, lymphatic drainage into the pleura with granulomatous pleuritis and effusions, or to bronchi with bronchocentric granulomatous lesions (Fig. 6-54) or tuberculous bronchopneumonia. Granulomas also may encroach upon blood vessels, mimicking a “granulomatous” vasculitis. The hemophagocytic syndrome, which has been implicated in a variety of bacterial, viral, and parasitic infections, also has been associated with tuberculosis.150
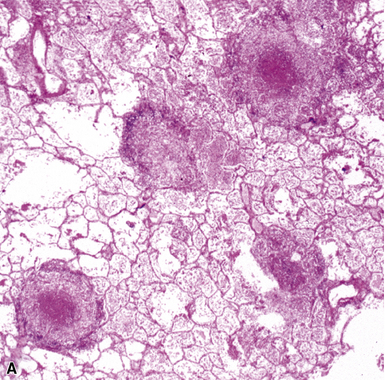
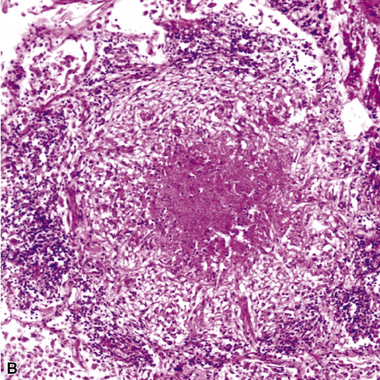
Figure 6-53 Miliary tuberculosis.
A, Miliary pattern. B, Epithelioid granulomas with necrotic central zones.
Post-primary Tuberculosis
Post-primary tuberculosis, the most common form in adults, typically involves the apices of the upper lobes, producing granulomatous lesions with greater caseation, often with cavities and variable degrees of fibrosis and retraction of the parenchyma.136 Fibrosis and bronchiectasis occurs with the healing of cavities and is the major cause of pulmonary disability in this disease.151 Recent studies have proposed that post-primary disease begins as a form of lipoid pneumonia, with bacilli-laden foamy alvolar macrophages and bronchiolar obstruction progressing to cavitary disease, as a result of caseation, and microvascular occusion due to delayed-type hypersensitivity.152 Extension to other lobes, hilar or mediastinal lymph nodes and miliary spread through the lungs and to extrapulmonary sites can complicate this form of disease. Other presentation patterns include acute and organizing diffuse alveolar damage with advanced or miliary disease, acute tuberculous bronchopneumonia, and the solitary pulmonary nodule (tuberculoma). A proximal endobronchial form may mimic a neoplasm and also is noteworthy for extensive necrosis and often large numbers of bacilli.153 Because characteristic granulomatous morphology may not be visible around the necrotic material, stains for mycobacteria should be considered for all necrotic endobronchial samples.
Nontuberculous Mycobacterial Infections
NTM infections may be similar to those due to M. tuberculosis, but certain differences have been noted. For example, the NTM pathogens do not cause the same sequence of primary or post-primary disease, and systemic dissemination does not occur except in the immunocompromised patient. M. kansasii is more virulent than MAC, and the infection-associated histopathologic pattern is more like that produced by M. tuberculosis.154
Infections due to MAC and other common pulmonary NTM pathogens generally manifest as one of five clinicopathologic entities: solitary pulmonary nodule, chronic progressive pulmonary disease, disseminated disease, chronic bronchiolitis with bronchiectasis, and hypersensitivity-like pneumonitis.134,155 Solitary pulmonary nodules generally exhibit granulomas resembling those caused by M. tuberculosis.
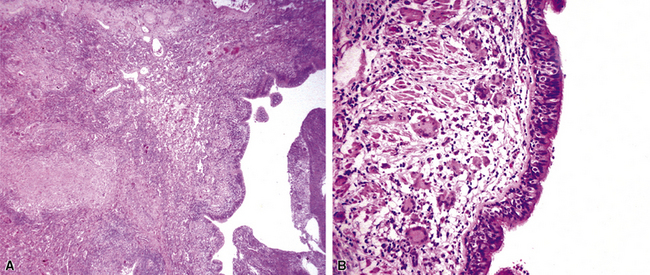
Chronic progressive disease also resembles tuberculosis, with upper lobe thin-walled cavities and granulomatous inflammation, with or without caseous necrosis (Fig. 6-55). Multiple confluent granulomas in fibrosis can mimic sarcoidosis. Organisms usually are sparse and more diffiult to find in the immunocompetent patient. This presentation most often is seen in patients with underlying chronic lung disease such as COPD, bronchiectasis, cystic fibrosis, pneumoconiosis, reflux disease, or pre-existing cavitary lung disease of any cause (including old tuberculous cavities).
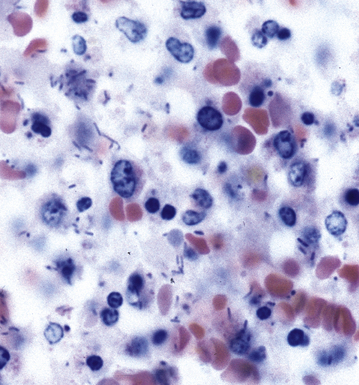
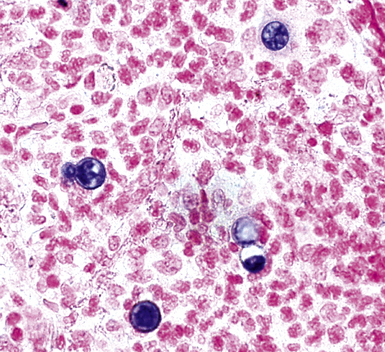
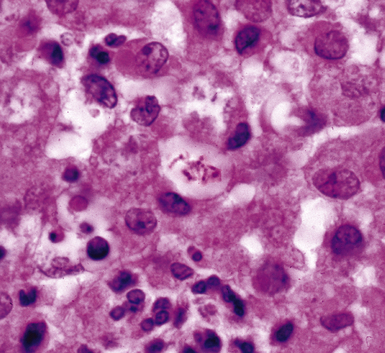
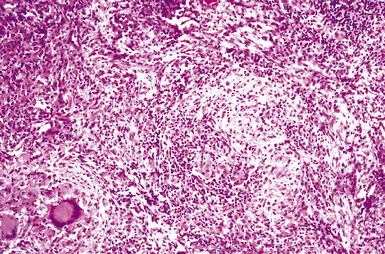
Disseminated disease typically is associated with the immunocompromise produced by HIV infection, in which the disease tends to target the gastrointestinal tract (the likely portal of entry), and pulmonary and reticuloendothelial disease signifies dissemination.156 In this setting, NTM bacilli (predominantly MAC) proliferate characteristically to high levels in poorly formed granulomas, or in sheets and clusters of plump, finely vacuolated macrophages (“pseudo-Gaucher” cells) containing abundant phagocytosed intracytoplasmic bacilli (Fig. 6-56).
A distinctive form of NTM disease occurs as the “Lady Windermere syndrome.” In the classic clinical scenario, an elderly, nonsmoking, immunocompetent woman of particular habits, demeanor, and body type presents with multiple pulmonary nodules, preferentially involving the middle lobe and lingula. The airway-centric granulomas and bronchiectasis can be subtle or pronounced (Fig. 6-57); this has been recognized as one of the patterns of middle lobe syndrome.157 NTM bacilli also can colonize bronchiectatic lung from any cause, with resultant granulomatous inflammation predominantly affecting the airway walls—presumably a result of localized decreased mucociliary clearance.
Hypersensitivity-like pulmonary disease recently has been associated with contaminated water in hot tubs (“hot tub lung”) and other environmental sources such as humidifiers and air conditioners.17 Biopsy reveals a miliary bronchiolocentric and interstitial granulomatous pattern, similar to that produced by hypersensitivity pneumonitis (Fig. 6-58). A similar infection-colonization-hypersensitivity syndrome has been described in workers exposed to metal-working fluid aerosols.158 The clinical, radiologic, and pathologic findings are similar to disease associated with hot tub use and other water sources except that a distinctive rapid-growing NTM species, M. immunogenum, has been recovered almost exclusively. Organisms are difficult to find in these cases but sometimes can be recovered in culture or with molecular techniques. Whether this entity represents an infection, a colonization, a hypersensitivity reaction, or a hybrid condition remains unresolved at this time.
A rare morphologic manifestation of mycobacterial infection is the so called “spindle cell inflammatory pseudotumor” (Fig. 6-59) which may occur in lung, skin, lymph nodes, and a number of other sites in immunocompromised patients.159 The etiologic agents usually are NTM (MAC and M. kansasii), but M. tuberculosis has also been identified in some cases. Another uncommon variant is proximal endobronchial disease, discussed earlier in the spectrum of post-primary tuberculosis. Most cases are due to M. avium complex and manifest as polypoid lesions in immunocompromised HIV-infected patients, but this lesion also may be seen in immunocompetent persons.160
Certain species of rapidly growing mycobacteria (RGM) are capable of producing pulmonary disease, albeit infrequently.132,161 Nevertheless, M. abscessus is the third most frequently recovered NTM respiratory pathogen in the United States, after M. avium complex and M. kansasii. It accounts for 80% of respiratory tract isolates, making it the leading rapidly growing mycobacterial species recovered from the lung. M. abscessus produces chronic lung infection that has a striking clinical and pathologic similarity to M. avium complex infection, including the propensity to involve the lungs of patients with bronchiectasis. The RGM also have been thought to colonize lipoid pneumonia162; however, it is more likely that the pathogenesis of the lung injury pattern caused by the RGM is similar to that seen in skin and soft tissue cases, in which various combinations of suppurative foci, poorly formed or necrotizing granulomas, scattered multinucleated giant cells, and vacuoles are typical (termed “pseudocysts”).163 These combined features may mimic lipoid pneumonia and constitute an important clue to the presence of RGM infection.
Cytopathology
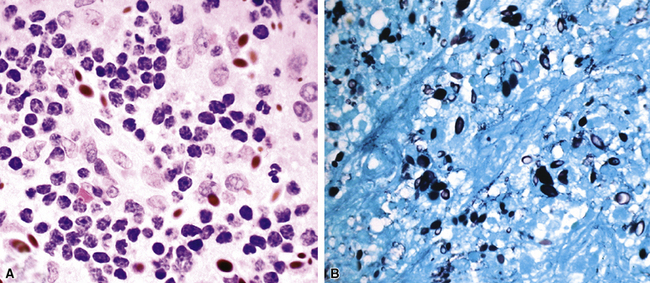
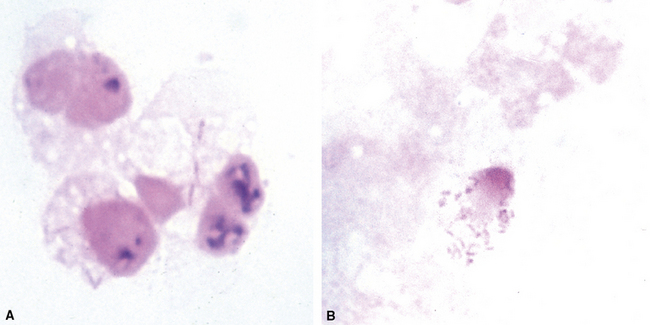
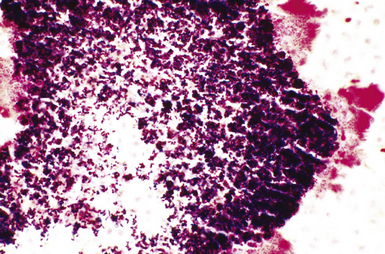
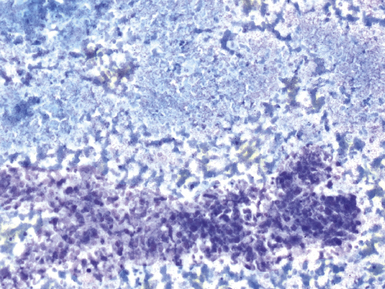
Fine-needle aspiration biopsy has been successfully used to diagnose both tuberculous pulmonary lesions and nontuberculous mycobacterial infections.164 The finding of finely granular amorphous necrotic debris associated with aggregates of epithelioid histiocytes (with or without multinucleate giant cells) (Fig. 6-60) is suggestive of a mycobacterial or fungal infection.165 In this setting, necrotic cancers must be excluded by a thorough search for atypical cells.
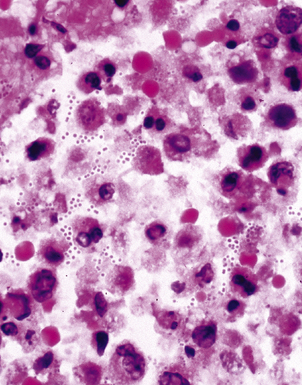
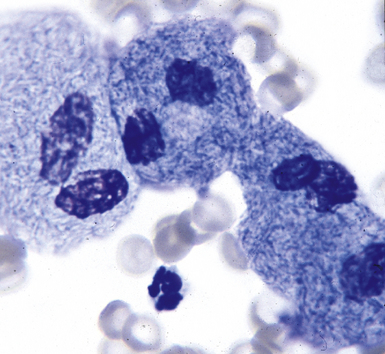
Special stains for acid-fast bacilli can be applied to aspirate smears, but culture of the aspirate is more likely to yield the etiologic agent when bacilli are sparse. Also, culture is still necessary for species identification and, if necessary, antimicrobial susceptibility testing. Epithelioid granulomas manifest a similar cellular pattern, but the granular necrotic debris is absent. Another pattern that may be seen, particularly in specimens from the immunocompromised patient, is a pure histiocytic or macrophage reaction with few or no epithelioid or multinucleate giant cells or necrotic debris. Numerous bacilli may be present in the distended cytoplasm of histiocytes and in the extracellular background. In air-dried (Diff-Quik) and alcohol-fixed (H&E- or Papanicolau-stained) smears, the bacilli may be recognized as negative images (Fig. 6-61).
Microbiology
The traditional as well as newer molecular approaches to the laboratory diagnosis of mycobacterial lung infection are outlined in Box 6-11. The mycobacterium is a slender but slightly curved bacillus, 4 μm in length, often with a beaded appearance; the length, curvature, and beadedness sometimes are accentuated in M. kansasii.166 In tissue sections or on smears, the Ziehl-Neelsen acid-fast stain or auramine-rhodamine fluorescent stains are most often recommended for best visualization. Organisms most often are found within the area of granulomatous reaction at the immediate periphery of the necrotic zone of the granulomas, or the cellular reactive process in the lining of cavities. Sections from several tissue blocks may be required to find organisms. Bacilli are rarely found in the absence of necrosis, except in smears from immunocompromised patients, in which they are visible and abundant within pseudo-Gaucher cells on H&E-stained sections, or as ghosted intracellular outlines with Giemsa-type stains. Dead bacilli lose their acid-fast character but sometimes may be identified with the GMS stain. The NTM, especially the RGM, may be more sensitive to acid alcohol decoloration and may not stain well or at all with the auramine-rhodamine method.132 A commercial immunohistochemical reagent for mycobacteria is now available but is effective only in cases in which traditional acid-fast stains yield a positive result.32 The differentiation of mycobacterial species in Ziehl-Neelsen–positive, formalin-fixed sections also has been achieved by in situ hybridization techniques with specific nucleic acid probes.167–169 PCR amplification plus identification is likely to be the most sensitive technique in those cases in which the lesion is suspected to harbor mycobacteria but yields a negative result on acid-fast staining.170 This technique may also be useful in cases in which the characteristic granulomatous pattern of inflammation is lacking, or mycobacteria have been identified in acid-fast–stained sections but culture results remain negative or cultures were not performed.171,172
Conventional wisdom states that culture is more sensitive than direct examination; however, the literature clearly documents cases in which acid-fast stains on tissue biopsies succeeded when cultures of tissue failed—an outcome that speaks to the virtue of perseverance in the face of compelling histopathologic findings.173 Furthermore, tissue culture is prone to sampling error unless more than one site is sampled.174 Specimens also may be smear positive and culture negative in patients whose disease has been treated. When only a rare bacillus is found, a strict criteria must be maintained and artifactual “pseudo” acid-fast bacilli excluded. As a general rule, a cutoff value of three organisms for a positive result seems prudent. False-positive smears also can result from contamination with local tap water, which may harbor mycobacteria.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree