4

Lung Biopsy, Lung Resection, and
Autopsy Lung Specimens: Handling
and Diagnostic Limitations
Lung Biopsy
In most surgical pathology practices, lung biopsies, especially transbronchial biopsies, are common specimens, but there is more than a little confusion among both clinicians and pathologists about the proper handling of biopsy specimens and the diagnostic limitations inherent in different types of lung biopsy. This section of the chapter covers transbronchial and open/thoracoscopic biopsies with a focus on what types of diseases can and cannot be diagnosed and how the specimen should be prepared. Cutting needle biopsy is not discussed because it is used in relatively few institutions, but review data on experience with this technique can be found elsewhere.1 Cytologic techniques are covered in Chapter 27 and pleural biopsy results in Chapter 26.
No matter how knowledgeable the pathologist, it is crucial to correlate pathologic features with clinical findings for the proper interpretation of lung biopsies. Apart from tumors, many pulmonary processes as seen on biopsy have relatively non-specific (and sometimes misleading) pathologic features. The clinical significance of honeycombing, for example, may vary from none, if a biopsy has chanced upon a localized old scar, to a part of usual interstitial pneumonia (UIP), to the end stage of a wide variety of interstitial lung diseases (discussed later in the chapter). Histologically there may be no way to make the distinction, and in such instances the correct diagnosis can only be arrived at by the addition of clinical information. In recent years the correct diagnosis of lesions with relatively nonspecific morphologic features has become even more crucial as new classifications of idiopathic interstitial pneumonias2 (see Chapter 20) have defined conditions with good and poor prognoses, conditions that require different therapies. Fortunately, at the same time the widespread use of high-resolution computed tomography (CT) has enabled the radiologist and respirologist to greatly narrow the differential diagnoses, so that in most instances simply asking the respirologist for a differential diagnosis will provide the pathologist with an excellent guide to the likely choices of pathologic diagnosis.
Open and Thoracoscopic Lung Biopsy
Open lung biopsy is the traditional method of obtaining large pieces of lung tissue for diagnosis. Over the past 10 years, many institutions have adopted the use of thoracoscopic lung biopsy because of its relative ease and lower morbidity. Pathologic comparisons between open and thoracoscopic biopsies suggest that, provided adequate-sized pieces of tissue are obtained (discussed later in the chapter), they provide very comparable diagnostic yields and pathologic appearances.3–7 For convenience, this chapter often refers to “open biopsy,” which is meant to apply to both and thoracoscopic biopsy, except as noted.
1. Receive specimen fresh in surgical pathology laboratory. 2. Take material for culture from edge of biopsy (unless you are certain that an infectious process is not involved, or culture has been taken in operating room). 3. Inflate specimen with fixative using a small needle. 4. Fix for 30 to 60 minutes, then block to give the largest possible face. 5. Avoid frozen section if possible, but if a frozen section is absolutely necessary, inflate the portion of the biopsy to be frozen with frozen section embedding medium, then freeze and cut. Remainder of biopsy can be inflated with ordinary fixative. 6. Insist on a good-sized biopsy (minimum 2 cm on a side) and preferably more than one biopsy. |
Handling and Artifacts
Table 4–1 lists the important steps in handling an open or thoracoscopic biopsy. Once removed from the chest, lung tissue tends to collapse, and collapse can be a major problem in biopsy interpretation, a problem that in severe cases may render a biopsy totally unreadable. The major problem with parenchymal collapse is that it creates mimics of interstitial inflammation and fibrosis (Fig. 4–1) in part because alveolar walls are elastic and behave somewhat like rubber bands (if stretched, they become thinner, and if allowed to contract, thicker), and in part because, in uninflated specimens, the alveolar walls tend to collapse on each other. Focal parenchymal collapse may even produce an appearance that mimics a mass lesion or neoplasm (Fig. 4–2). Some notion of whether disease is actually present may be obtained by finding an area of the biopsy that is definitely normal and tracing the alveolar walls into the apparently abnormal areas. If the individual walls can be clearly followed and do not appear to change, the biopsy is probably normal. Reticulum or elastic tissue stains may be of some help in this situation if they demonstrate that several independent juxtaposed alveolar walls are present. However, it is often difficult and sometimes impossible to determine whether interstitial disease is actually present in uninflated biopsies, and other processes may occasionally be hidden in the mass of collapsed parenchyma. Closely associated with parenchymal collapse is the formation of bubble-like spaces. These are of no particular importance in themselves but do serve to alert the pathologist to the presence of considerable collapse.
The solution to this problem is to fix open and thoracoscopic biopsies in inflation. For open lung biopsies one can have the anesthesiologist inflate the lung immediately before the biopsy; the surgeon then applies the clamps or staple gun to the inflated piece of lung, resects the specimen, and immediately drops the resected piece, still held in inflation, into fixative.8 This technique provides good results but requires coordination among anesthesiologists, surgeons, and pathologists, which may be difficult to achieve in practice. It also is not satisfactory for thoracoscopic lung biopsies, in which the lung is collapsed for inspection, and inflation could interfere with removing the tissue from the thorax.4
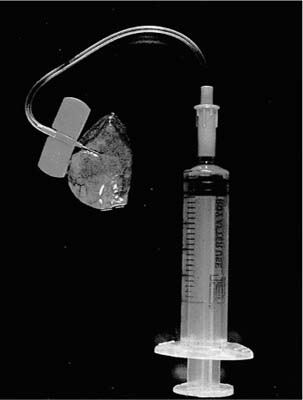
An alternate, and generally preferable, procedure is to receive the specimen fresh in the surgical pathology laboratory and to inflate it with fixative.9 The technique is simple and quick: a small (~25-gauge or smaller) needle attached to a fixative-filled syringe is inserted into the parenchyma through the pleura or through a cut surface and fixative is gently instilled until the lung is expanded (Fig. 4–3). Fixative will escape from any cut surface, but this does not matter because the important point is to expand the parenchyma; once held in expansion for a few minutes, the parenchyma will not collapse. If the biopsy happens to include an interlobular septum, it may be necessary to inflate two different areas to achieve complete expansion. The inflated specimen is then put into fixative for 30 to 60 minutes, and finally cut and allowed to fix for a few hours or overnight. Inflation sometimes produces a degree of edema in the interlobular septa, particularly if the specimen is overinflated, but this artifact does not interfere with interpretation and is worth accepting, given the far superior ease of interpretation of the important parts of the biopsy (Fig. 4–1). More details of the method are provided elsewhere.9 Unfortunately, the syringe inflation technique does not work on partially fixed specimens, so unless one is sure that the surgeon will inflate and fix the lung properly, it is probably best to insist that all lung biopsies immediately be brought fresh to the surgical pathology laboratory.
Regardless of the method used to produce inflation, proper orientation of the cut specimen is crucial because many lung diseases require interpretation of low power architecture. Thus the specimen should always be blocked to give the largest possible face for histologic examination.
Unless one is dealing with a definite malignancy, lung biopsies should always be cultured. This is best performed in the operating room, but if necessary, a piece of the edge of the specimen can be taken for culture when it is received in the surgical pathology laboratory. Additional pieces can be removed for electron microscopy, specialized immunohistochemistry, or molecular biologic examination. We do not recommend that frozen sections of open and thoracoscopic lung biopsies be performed unless an immediate diagnosis is absolutely necessary, because collapse artifacts are hard to avoid in frozen sections and frozen section thickness variations can also produce false impressions of interstitial inflammation. However, if a frozen section is necessary, the procedure described by Gianoulis et al10 can be followed: instead of fixative, the biopsy specimen is inflated with frozen section embedding medium (OCT or a similar product), diluted 50% with saline if the original is too thick to go through a needle. It is then sliced, frozen, and cut. If the biopsy is reasonably large, one can cut it in half (again blocking to give the largest possible face) and inflate half with embedding medium for frozen section and half with fixative for routine section.
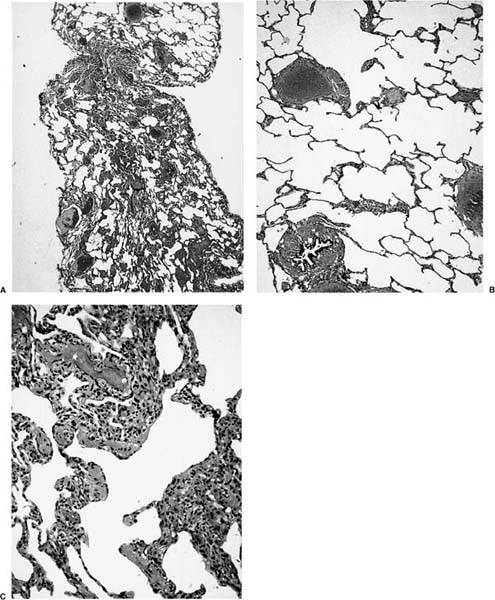
FIGURE 4–1 Open lung biopsy showing how collapse artifacts produce a false impression of interstitial fibrosis and inflammation. This particular biopsy was pinched during the surgical procedure, so that part of the biopsy has remained inflated and part has collapsed (A). The inflated part (B) shows obviously normal parenchyma, whereas the collapsed part (C) mimics a chronic interstitial pneumonia.
In nonimmunocompromised patients hematoxylin and eosin (H&E) stains are sufficient for most situations. Elastic stains are useful to specifically examine vascular abnormalities, and iron stains may be useful in providing evidence of old hemorrhage. We do not recommend the use of trichrome stains to demonstrate interstitial fibrosis. Trichrome stains always show interstitial collagen, but unless one routinely examines normal lungs with trichrome stains, it is difficult to distinguish minor degrees of fibrosis from normal. Marked fibrosis is usually quite obvious without a trichrome stain. For specimens from immunosuppressed patients, stains for organisms (Gram, acid-fast, methenamine-silver) should always be prepared (discussed later in the chapter).
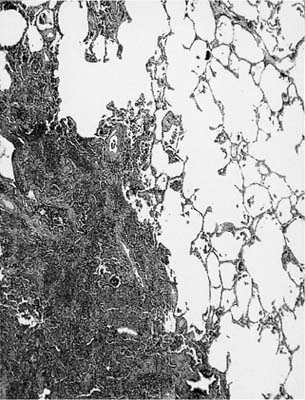
FIGURE 4–2 Severe collapse artifact in an open biopsy producing a false impression of a mass lesion.
FIGURE 4–3 Simple device for inflating open and thoracoscopic lung biopsies with fixative before slicing.
Apart from collapse, a variety of other histologic artifacts may crop up in these types of biopsies. Occasionally distinguishing surgical hemorrhage from true alveolar hemorrhage can be a problem; this is best approached by looking for evidence of hemosiderin-laden macrophages as an indication that the hemorrhage has been present for some time, and iron stains are useful in this setting. Again, some familiarity with normal is essential, because macrophages that stain weakly for iron are common in the lungs of smokers (“smoker’s macrophages”). Neutrophil margination may be seen in large and small vessels if the lung was handled extensively or clamped for a long time before biopsy, and both hemorrhage and margination appear to be more common in thoracoscopic biopsies.4 Manipulation of the lung during the biopsy process can sometimes produce a peculiar picture of overexpansion of alveoli that resembles emphysema;4 if one remembers that emphysema is not a useful diagnosis in an open or thoracoscopic biopsy (discussed later in the chapter), the problem disappears.
Size, Site, and Number of Biopsies
The major advantage of open and thoracoscopic lung biopsy, compared with transbronchial biopsy, resides in the relatively large amount of tissue available to the pathologist. In general, small biopsies of any type are more likely to be difficult to diagnose or nondiagnostic (as described later in the chapter, some types of interstitial lung disease cannot be diagnosed at all on small biopsies), and it is up to the pathologist to insist on an adequate sized biopsy. A good rule of thumb is that these types of biopsy should measure at least 2 cm on a side. Obtaining large pieces in general is technically easy; in a study by Kadokura et al4 comparing the results of open and thoracoscopic biopsies, the mean greatest diameter of the specimen was 4.1 cm for thoracoscopic biopsies and 4.2 cm for open biopsies, with the largest biopsies greater than 10 cm in diameter.
The site of biopsy is just as important as the handling of the specimen. Where the biopsy should be performed varies with the nature of the lesion. Obviously, biopsies should be taken directly from suspected tumor masses, and CT scans in general provide a good guide to sampling localized lesions. However, some surgeons apply this same principle to lungs with diffuse interstitial disease and biopsy the area that feels or looks the worst. Frequently such a biopsy yields only nonspecific honeycombing (discussed later in the chapter), a finding that renders the biopsy worthless. In this situation, the best area to biopsy is one that is intermediate between worst and best5 or, alternately, take more than one biopsy; unless the patient is a poor operative risk, the small amount of extra time needed to obtain two or three biopsies is well worthwhile.
Biopsies should not be taken from the tips of the lobes, particularly the lingula and right middle lobe tips. These dependent areas may show pathologic features completely different from those seen in the rest of the lung, especially vascular abnormalities and interstitial fibrosis and inflammation, even when the rest of the lobe is completely normal.11,12 If presented with such a biopsy, the pathologist should be extremely cautious in making a diagnosis of any type of acute or chronic diffuse interstitial pneumonia or vascular disease. Note that this caution is not meant to completely prevent the use of lingular biopsies, as some apparently believe,13 but only to suggest that taking the biopsy a few centimeters away from the lingular (right middle lobe) tip avoids nonspecific artifacts.
Specific and Nonspecific Diagnoses
Open biopsy allows the pathologist to examine a large area at low power and to find either a low-power pattern that is potentially diagnostic despite the lack of any one specific feature, for example, in UIP, or to find a lesion that is specific but scattered through the parenchyma, for example, the lesions of eosinophilic granuloma (Langer-hans cell histiocytosis). However, even in open and thoracoscopic biopsies, some morphologic patterns have little significance. Thus conceptually one can divide possible diagnoses into four broad categories:
1. The histopathologic features provide high morphologic specificity. Obvious examples are malignancies, eosinophilic granuloma, and sarcoidosis or any other lesion where a particular specific morphologic finding is diagnostic. These diseases are considered in detail in other chapters.
2. The histologic features are nonspecific, unreliable, or misleading in the context of a biopsy. Emphysema and honeycombing fall into this category. A diagnosis of emphysema really requires the inflated whole lung or lobe. Accurate assessment of the presence and type of emphysema is usually not possible on the basis of an open or thoracoscopic lung biopsy, and certainly no judgment of its severity is possible on such evidence. More to the point, however, no one performs a biopsy to diagnose emphysema. Thus even if emphysema is really present in an open or thoracoscopic biopsy, but no other abnormality is found, it is likely that the biopsy has missed the lesion of importance.
Honeycombing is a term used by both radiologists and pathologists to refer to end-stage lung disease. Honeycombing consists of a variable combination of thick-walled cysts (as opposed to the thin-walled cysts of emphysema), which may range from a few millimeters to a few centimeters in diameter, and intervening solid fibrous tissue (Fig. 4–4) (see also Chapter 20 for further discussion). Microscopically, the walls of the cystic spaces usually show prominent fibrous tissue (Fig. 4–5), but in some instances there is considerable muscular metaplasia (“muscular cirrhosis of the lung” is an old name for honeycombing). Bronchial epithelial metaplasia and type II cell metaplasia are also common in the epithelium lining the cystic spaces, and the spaces themselves frequently contain acute and chronic inflammatory cells, occasionally giant cells, and mucus. The inflammatory findings have no significance; in particular, they generally do not represent a diagnosable infection, and they do not represent a specific inflammatory lung disease.
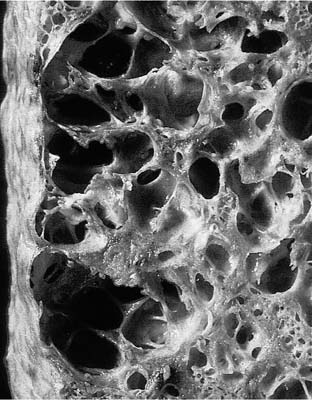
FIGURE 4–4 Gross appearance of honeycombing. This example is from a case of usual interstitial pneumonia, but honeycombing can be seen in many different types of lung disease and is not, in itself, a specific diagnosis.
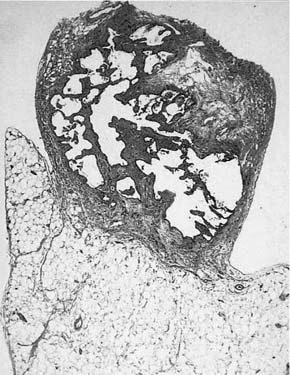
FIGURE 4–5 Microscopic appearance of a lung biopsy showing only honeycombing (the lung tissue is adherent to chest wall fat). No specific diagnosis is possible from this biopsy.
Histologically, it is easy to decide that honeycombing is present in a biopsy, but honeycombing is a common end stage of many processes (Table 4–2) and thus is, in and of itself, of little diagnostic significance. In addition to specific diffuse diseases, localized scars of many causes can appear as honeycombed lung. If an open lung biopsy shows only honeycombed lung (Fig. 4–5), it is impossible to determine from the microscopic appearances what the underlying disease (if any) might be. This is not to say that the finding of honeycombing is entirely useless. Because honeycombing is a nearly universal finding in UIP,2 and because it is peripheral and thus easily biopsied in UIP, a biopsy showing honeycombing in a patient with classic clinical and radiographic evidence of UIP provides some support, albeit indirect, for that diagnosis. But if the underlying disease is not clear from the clinical and radiographic findings, then the finding of honeycombing alone should be reported as nonspecific.
Idiopathic interstitial pneumonias |
Usual interstitial pneumonia |
Desquamative interstitial pneumonia |
Nonspecific interstitial pneumonia |
Lymphocytic interstitial pneumonia |
Extrinsic allergic alveolitis |
Adult respiratory distress syndrome |
Radiation injury |
Pneumoconioses, for example, asbestosis |
Eosinophilic granuloma of lung |
Sarcoidosis |
Old infections, for example, tuberculosis |
Old nonspecific localized scars |
3. The histologic features do not show any one specific feature that allows the diagnosis; rather, it is the lower power architecture and combination of fairly nonspecific patterns in concert with the proper clinical and radiographic setting that create the diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree