Chapter 60 Lower-Extremity Ulceration
Ulceration of the lower extremity is a common condition that causes significant discomfort and disability.1 An ulcer is defined as a disruption of the skin with erosion of the underlying subcutaneous tissue. This breach may extend further to the contiguous muscle and bone. The pathophysiological mechanisms underlying ulcer formation are multifactorial and include neuropathy, infection, ischemia, and abnormal foot structure and biomechanics. It is not surprising then that management of the diabetic foot is a complex clinical problem requiring an interdisciplinary approach.2,3 Minor trauma, often footwear related, is a frequent inciting event. A chronic ulcer is defined as a full-thickness skin defect with no significant reepithelialization for more than 4 weeks.
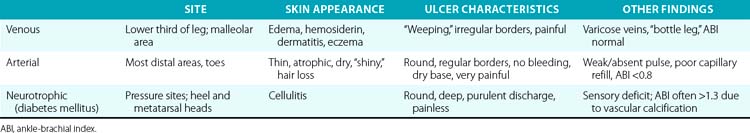
Three etiologies of leg ulcerations are responsible for almost 95% of leg ulcers: about 40% to 80% are due to underlying venous disease, 10% to 20% are due to arterial insufficiency, and 15% to 25% are secondary to diabetes mellitus; in 10% to 15% of patients, a combination of two or more causes exists. Prolonged pressure and local infection are common causes of leg ulcers with minimal vascular compromise. Rare causes are responsible for less than 5% of all leg ulcers4 (Box 60-1). The disease entities that usually underlie leg ulceration (e.g., venous insufficiency, peripheral artery disease (PAD), diabetes mellitus) are associated with significant patient morbidity and mortality. A detailed knowledge of the clinical picture, pathogenesis, relevant diagnostic tests, treatment modalities, and differential diagnosis of leg ulcerations is essential in planning the optimal treatment strategy (Table 60-1). An incorrect or delayed initial diagnosis may harm the patient and increase the risk of serious complications, including permanent disability and amputations.
![]() Box 60-1 Causes of Lower-Extremity Ulcers
Box 60-1 Causes of Lower-Extremity Ulcers
The exact prevalence of lower-extremity ulcers in the United States is unknown. The prevalence of leg ulceration in the general population of Western nations is 1% to 3.5%, with the prevalence increasing to 5% in the geriatric population.5–8 Data from these studies most likely underestimate the true prevalence because they do not include patients with leg ulcers who are not known to the healthcare system.
The cost of treating leg ulceration is staggering. Epidemiological studies from Sweden estimated annual costs of treatment of lower-extremity ulcers at $25 million. In England, the estimated cost of care for patients with leg ulcers in a population of 250,000 is about $130,000 annually per patient.9 Items factored into the equation include physician visits, hospital admissions, home health care, wound care supplies, rehabilitation, time lost from work, and jobs lost. Adding to the cost is the chronic nature of these wounds, high rate of recurrence, and propensity for infection. A true accounting of the cost is difficult because of the unknown prevalence of disease.
Because the disease affects a patient’s lifestyle and attitude, the social cost of leg ulcers accrue. The ability to work may be temporarily or permanently affected by the condition,8 and the reduction in work capacity adds to the medical cost to society. An estimated 10 million workdays are lost from lower-extremity ulcers in the United States annually, and this figure may be low.10,11 A report in 1994 focused on the financial, social, and psychological implications of lower-extremity lesions in 73 patients.12 Among the study patients, 68% reported feelings of fear, social isolation, anger, depression, and negative self-image because of the ulcers. In addition, 81% of the patients felt that their mobility was adversely affected. Within the younger population that was still actively working, there was a correlation between lower-extremity ulceration and adverse effect on finances, time lost from work, and job loss. In addition, there was a strong correlation between time spent on ulcer care and feelings of anger and resentment. These factors combined to have a negative emotional impact on their lives.
Biomechanics of Walking and Ulcer Formation
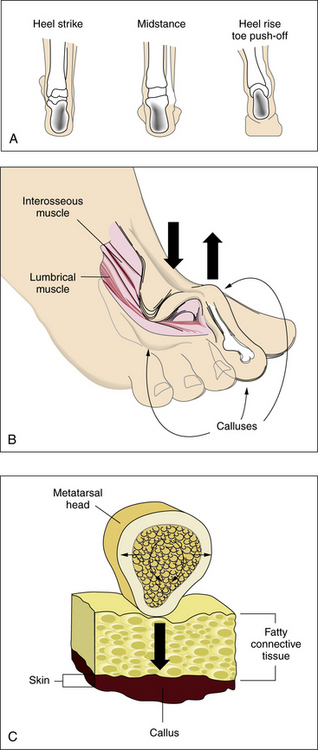
An appreciation of the biomechanics required for walking is essential to understanding the etiology of foot ulcers. The foot is a complicated biological structure containing 26 bones, numerous joints, and a network of ligaments, muscles, and blood vessels. Gait is a complex set of events that requires triplanar foot motion and control of multiple axes for complete bipedal ambulation13 (Fig. 60-1A). When the heel hits the ground, its outer edge touches first; the foot is in a supinated position, which makes it firm and rigid. The soft-tissue structures (muscles, tendons, and ligaments) then relax, allowing the foot to pronate. The foot becomes less rigid and is able to flatten, absorb the shock of touchdown, and adapt to uneven surfaces. During midstance, the heel lies below the ankle joint complex, the front and back of the foot are aligned, and the foot easily bears weight. Toward the end of midstance, the soft-tissue structures begin to tighten; the foot resupinates and regains its arch. The foot is again firm, acting as a rigid lever for propulsion. The heel lifts off the ground, swings slightly to the inside, and the toes push weight off the ground.
Sensory input from the visual, vestibular, and proprioceptive systems is necessary to modify learned motor patterns and muscular output to execute the desired action. Various external and internal forces affect foot function.14 The combination of body weight pushing down and ground reactive force pushing up creates friction and compressive forces. Shear results from the bones of the foot sliding parallel to their plane of contact during pronation and supination. Foot deformities or ill-fitting footwear enhance pressure points because they focus the forces on a smaller area. When the foot flattens too much or overpronates, the ankle and heel do not align during midstance, and some bones are forced to support more weight. The foot strains under the body’s weight, causing the muscles to pull harder on these areas, making it more difficult for tendons and ligaments to hold bones and joints in proper alignment. Over time, swelling and pain on the bottom of the foot or near the heel may occur. Bunions can form at the great toe joint, and hammertoe deformities can form at the lesser toes. Abnormal foot biomechanics resulting from limited joint mobility and foot deformities magnify shearing forces, resulting in increased plantar pressure on the foot during ambulation (see Fig. 60-1B-C). This can represent critical causes for tissue breakdown.
Pathophysiology of Ulcer Formation
Venous Disorders
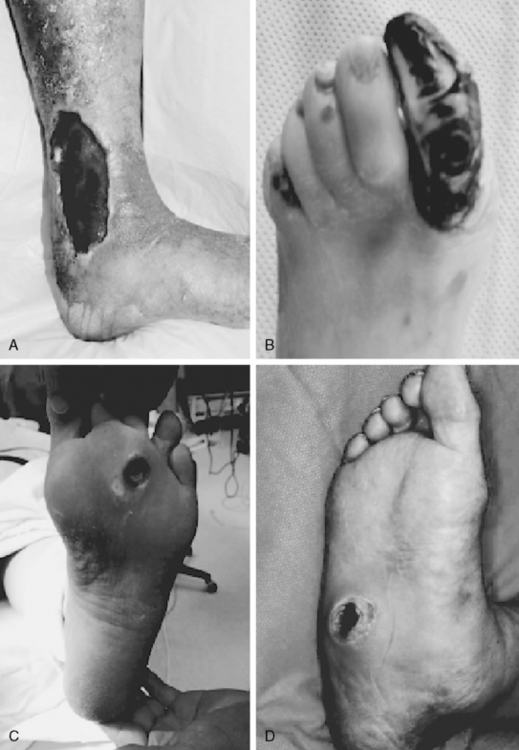
Venous leg ulcers are the most frequently occurring chronic lower-extremity wounds (Fig. 60-2A) (also see Chapter 56). The prevalence of lower-extremity ulceration resulting from chronic venous disease (CVD) in European and Western populations is estimated to be 0.5% to 1%. In the United States, it is estimated that between 600,000 and 2.5 million patients have venous ulcerations; treatment costs are estimated at $2.5 to $3 billion dollars, with a corresponding loss of 2 million workdays per year.15 Ten years ago, the estimated annual cost of treatment for venous ulcer patients was almost $40,000 per patient.16 This cost has risen since then. The pathophysiology of venous ulceration is straightforward. Blood returns from the lower extremities against gravity to the inferior vena cava (IVC) through the deep and superficial venous systems. The deep veins are located within the muscles and deep fascia of the legs. The superficial system consists of the great saphenous vein and the small saphenous vein and is located within the subcutaneous fat. Valves are present within all three systems and prevent retrograde flow of blood. A portion of blood from the superficial system is directed to the deep system through the communicating perforators. While standing, about 22% of the total blood volume is localized to the lower extremities, and hydrostatic pressure in the foot veins can reach 80 mmHg. In healthy individuals with competent venous valves, the efficient calf muscle pump can reduce venous pressure by two thirds during exercise. Venous insufficiency occurs when any of these elements do not function adequately. Pressure in the venous system increases, and (most importantly) ambulatory venous pressure rises during leg exercise. The primary cause of venous hypertension is insufficiency of the valves of the deep venous system and perforating veins of the lower leg.
The exact mechanism by which ulcerations develop in patients with venous insufficiency is not clear. One theory is that ulceration is due to increased intraluminal pressure within the capillary system of the leg. The capillaries become dilated and elongated, and blood flow is sluggish, resulting in microthrombi formation and frequently leading to capillary occlusion. Fibrin, albumin, and various macromolecules leak into the dermis, where they bind to and trap growth factors, making them unavailable for the tissue repair process.17 Leakage of fibrinogen through capillary walls results in deposition of pericapillary fibrin cuffs,18 which has been suggested as a physical barrier impeding passage of oxygen.10 Iron deposition, white blood cell (WBC) accumulation, decreased fibrinolytic activity, and a myriad of inflammatory responses to vascular damage are all postulated to be the final pathways leading to venous ulcerations, but it is still not clear whether they represent causative factors.
Tissue hypoxia appears to be the major underlying factor in developing venous ulceration. Unlike ulcers associated with arterial insufficiency, this hypoxic state is not caused by decreased blood flow to the legs. Patients with venous insufficiency usually have adequate blood flow to their lower extremities. Direct measurements of transcutaneous oxygen levels on the lower leg have demonstrated that exercise produces a marked rise in skin oxygen tension in normal legs, but not in those affected by venous insufficiency. Exercise reduces venous pressure in patients with competent valves, thus removing the stimulus for reflex vasoconstriction. In patients with compromised valves, venous pressure remains high during exercise, and reflex vasoconstriction persists.19
Arterial Disease
The incidence of lower-extremity ulcers caused by PAD (see Fig. 60-2B) is increasing in Western nations.8 The general aging of the population and better diagnostic techniques may provide possible explanations for this observation. Risk factors for development of atherosclerotic lesions causing leg ischemia include diabetes mellitus, smoking, hyperlipidemia, hypertension, obesity, and age.20 Lack of perfusion decreases tissue resilience, leads to rapid death of tissue, and impedes wound healing (see Chapter 17). Wound healing and tissue regeneration depend on adequate blood supply to the region. Ischemia due to vascular disease impedes healing by reducing the supply of oxygen, nutrients, and soluble mediators involved in the repair process.21
The Diabetic Foot
Persons with diabetes mellitus are particularly prone to foot ulcers. The diabetic foot is a common and serious clinical condition that has its specific characteristics. The American Diabetes Association Consensus Group found that among persons with diabetes, the risk of foot ulceration was increased among men, patients who had had diabetes for more than 10 years, and patients with poor glucose control or cardiovascular, retinal, or renal complications.22 It is estimated that 15% of U.S. patients with diabetes will develop manifestations of diabetic foot disease in their lifetime.23,24 In this population, the prevalence of lower-extremity ulcers ranges from 4% to 10%, with an annual incidence of 2% to 3%.25 Although representing only 6% of the population, patients with diabetes account for 46%25 of the 162,000 hospital admissions for foot ulcers annually. Foot ulcers occur in up to 25% of patients with diabetes and precede more than 8 in 10 nontraumatic amputations. In 2005, approximately 1.6 million people were living with limb loss; this number is expected to more than double by 2050.26 Diabetic foot ulcers and their sequelae, amputations, are the major cause of disability, morbidity, mortality, and costs for these patients.23 Ulceration and infection of lower extremities are a leading cause of hospitalization in patients with diabetes.25 Treatment of pedal soft-tissue deficits in the diabetic patient population continues to be a medical and surgical challenge, thereby extending the length of their disability and significantly increasing the cost of medical care. Nearly half of all patients who undergo amputation will develop limb-threatening ischemia in the contralateral limb, and many will ultimately require an amputation of the opposite limb within 5 years. In 2000, the Centers for Disease Control and Prevention (CDC) estimated that 12 million Americans were diagnosed with diabetes, and the estimated annual direct and indirect costs of diabetes treatment in the United States was approximately $174 billion, with 1 in 5 diabetes dollars spent on lower-extremity care. Preventing ulcerations and/or amputations is critical from both medical and economical standpoints.2
Development of diabetic foot disease can be attributed to several primary risk factors, including neuropathy, ischemia, infection, and immune impairment. Four foot-related risk factors have been identified in the genesis of pedal ulceration: altered biomechanics, limited joint mobility, bony deformity, and severe nail pathology.22
Neuropathy
Neuropathy is the most common underlying etiology of foot ulceration and frequently involves the somatic and autonomic fibers. Although there are many causes of peripheral neuropathy, diabetes mellitus is by far the most common (see Box 60-1). Neuropathy is present in 42% of diabetic patients after 20 years27 and is usually a distal symmetrical sensorimotor polyneuropathy. Peripheral neuropathy is postulated to result from abnormalities in metabolism, one of which is a deficiency in sorbitol metabolism via the polyol pathway.28,29
Neurotrophic ulcers typically form on the plantar aspect of the foot at areas of excessive focal pressures. These are most commonly encountered over the bony prominences of the metatarsal heads and forefoot region because of the requirements of midstance and heel-off during the gait cycle (see Fig. 60-2C). Loss of protective sensation in the foot can lead rapidly to ulceration if patient education and preventive measures are not taken. Diabetic patients are especially prone to development of a neuro-osteoarthropathy known as Charcot foot.30 This condition is thought to involve autonomic nerve dysfunction that results in abnormal perfusion to foot bones, which leads to bony fragmentation and collapse (see Fig. 60-2D). The resulting “rocker-bottom foot” is prone to tissue breakdown and ulceration.23,30
Several investigators23,30,31 have demonstrated that there is an increase in both static and dynamic foot pressures.32 To date, high pressures alone have not been shown to cause foot ulceration. Rheumatoid patients with high plantar foot pressures but no sensitivity deficit have almost no evidence of foot ulceration.33
Type A sensory fibers are responsible for light touch sensation, vibratory sensation, pressure, proprioception, and motor innervation to the intrinsic muscles of the foot. Type C sensory fibers detect painful stimuli, noxious stimuli, and temperature. When these fibers are affected, protective sensation is lost. This manifests as a distal symmetrical loss of sensation described in a “stocking” distribution and proves to be the primary factor predisposing patients to ulcers and infection.34 Patients are unable to detect increased loads, repeated trauma, or pain from shearing forces. Injuries such as fractures, ulceration, and foot deformities therefore go unrecognized. Repeat stress to high-pressure areas or bone prominences, which would be interpreted as pain in the non- neuropathic patient, also go unrecognized. Sensory dysfunction results in increased shearing forces and repeated trauma to the foot.35,36 Patients have inadequate protective sensation during all phases of gait, so high loads are undetected owing to loss of pain threshold, which results in prolonged and increased forces.31,35 These problems manifest as abnormal pressure points, increased shearing, and greater friction to the foot. Because this goes unrecognized in the insensate foot, gait patterns remain unchanged, and the stresses eventually cause tissue breakdown and ulceration.
Motor neuropathy is associated with demyelinization and motor end-plate damage, which contribute to conduction defects. The distal motor nerves are the most commonly affected, resulting in atrophy of the small intrinsic muscles of the foot. Wasting of the lumbrical and interosseous muscles of the foot results in collapse of the arch and loss of stability of the metatarsal-phalangeal joints during midstance of the gait. Overpowering by extrinsic muscles can lead to depression of the metatarsal heads, digital contractures, and cocked-up toes; equinus deformities of the ankle; or a varus hindfoot.37
Autonomic involvement causes an interruption of normal sweating at the epidermal level and arteriovenous shunting at subcutaneous and dermal levels. Hypohidrosis leads to a noncompliant epidermis that increases the risk of cracking and fissuring. Arteriovenous shunting diminishes delivery of nutrients and oxygen to tissue regions, and skin and subcutaneous tissues become more susceptible to breakdown.38
Musculoskeletal deformities
Atrophy of the small muscles within the foot results in nonfunctioning intrinsic foot muscles referred to as an intrinsic minus foot39 (see Fig. 60-1B). Muscles showing early involvement are the flexor digitorum brevis, lumbricales, and interosseous muscles. These muscle groups act to stabilize the proximal phalanx against the metatarsal head, preventing dorsiflexion at the metatarsal phalangeal joint (MTPJ) during midstance in the gait cycle. With progression of the neuropathy, these muscles atrophy and fail to function properly. This causes the MTPJs to become unstable, allowing the long flexors (flexor digitorum longus and flexor hallucis longus) and extensors (extensor digitorum longus and extensor hallucis longus) to act unchecked on the digits. Dorsal contractures develop at the MTPJs, with development of hammer digit syndrome, also known as intrinsic minus disease.
The deformity acts to plantarflex the metatarsals, making the heads more prominent and increasing the plantar pressure created beneath them (see Fig. 60-1B). It also acts to decrease the amount of toe weight-bearing during the gait cycle, which also increases pressure on the metatarsal heads. In normal foot anatomy, a metatarsal fat pad located plantar to the MTPJs helps dissipate pressures on the metatarsal heads from the ground. When hammer digit deformity occurs, this fat pad migrates distally and becomes nonfunctional, resulting in elevated plantar pressures that increase the risk of skin breakdown and ulceration due to shearing forces.1
Overpowering by the extrinsic foot muscles also leads to an equinus deformity at the ankle and a varus hindfoot. A cavovarus foot type can develop, leading to decreased range of motion of the pedal joints, inability to adapt to terrain, and low tolerance to shock. In essence, a mobile adapter is converted to a rigid lever. Pressure is equal to body weight divided by surface area, thus decreasing surface area below a metatarsal head with concomitant rigid deformities and leading to increased forces or pressure to the sole of the foot. When neuropathic foot disease is associated with congenital foot deformities such as long or short metatarsals, a plantarflexed metatarsal, abnormalities in the metatarsal parabola, or a Charcot foot30 (see Fig. 60-2D), there is a higher propensity toward breakdown as a result of increased and abnormal plantar foot pressures.
Increased body weight and decreased surface area of contact of the foot components with the ground increase pressure. A low pressure but constant insult over an extended period can have the same ulcerogenic effect as high pressure over a shorter period. This is typical of the effect of tight-fitting shoes. If the magnitude of these forces in a given area is large enough, either skin loss or hypertrophy of the stratum corneum (callus) occurs (see Fig. 60-1C). Presence of callus in patients with neuropathy should raise a red flag because the risk of ulceration in a callused area is increased by two orders of magnitude.
Arterial disease
One of the major factors affecting diabetic foot disease is development of lower-extremity arterial disease.26 Peripheral artery disease is estimated to be two to four times more common in persons with diabetes than in others23,40 (see Chapter 14). Atherosclerosis occurs at a younger age in persons with diabetes than in others, and its hallmark is involvement of the tibioperoneal vessels, with sparing of the pedal vessels. In addition to being more prevalent in diabetes, atherosclerosis is more accelerated and results in a higher rate of amputations.41–43 Lesions in persons with diabetes tend to localize to the infracrural region. Relative sparing of the pedal vessels often assists pedal bypass. Occlusive lesions affecting the foot and precluding revascularization are not common in diabetic patients.23
Purely ischemic diabetic foot ulcers are uncommon, representing only 10% to 15% of ulcers in patients with diabetes. More commonly, ulcers have a mixed ischemic and neuropathic origin, representing 33% of diabetic foot ulcers.23 Initiation of an ischemic ulcer usually requires a precipitating factor such as mechanical stress. Ulcers often develop on the dorsum of the foot over the first and fifth metatarsal heads. A heel ulcer can develop from constant pressure applied while the heel is in a dependent position or during prolonged immobilization and bed rest. Once formed, the blood supply necessary to allow healing of an ulcer is greater than that needed to maintain intact skin. This leads to chronic ulcer development unless the blood supply is improved.
Infection
Patients with diabetes appear to be more prone to various infections than their nondiabetic counterparts.44 Several factors increase the risk of developing diabetic foot infections, including diabetic neuropathy, peripheral artery disease, and immunological impairment. Several defects in immunological response relate to increased infection risk in diabetics. Diabetic patients demonstrate a decrease in function of polymorphonuclear leukocytes that can manifest as a decrease in migration, phagocytosis, and decreased intracellular activity. Evidence suggests impaired cellular immune response as well as abnormalities in complement function.45,46 Some of the defects appear to improve with control of hyperglycemia.47
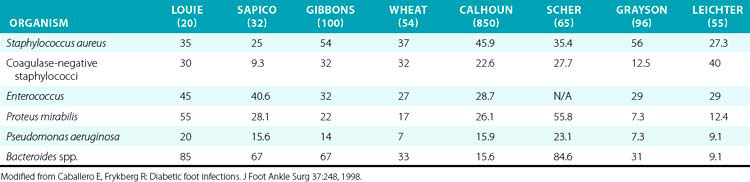
Undiagnosed clean neuropathic foot ulcers often convert to acute infections with abscess and/or cellulitis.48 Diabetic foot infections can be classified into those that are nonthreatening and those that are life or limb threatening. Non–limb-threatening diabetic foot infections are often mild infections associated with a superficial ulcer. They often have less than 2 cm of surrounding cellulitis and demonstrate no signs of systemic toxicity. These infections have on average 2.1 organisms48 (Table 60-2). Aerobic gram-positive cocci are the sole pathogens in 42% of these cases, with the most notable organisms being Staphylococcus aureus, coagulase-negative S. aureus, and streptococci. These less severe infections can often be managed with local wound care, rest, elevation, and oral antibiotics on an outpatient basis. A foot infection in a diabetic patient can present with a more severe, life- or limb-threatening picture. In these patients, there is usually a deeper ulceration or an undrained abscess, gangrene, or necrotizing fasciitis. Methicillin-resistant S. aureus (MRSA) is an increasingly common isolate.44 They tend to have greater than 2 cm of surrounding cellulitis, as well as lymphangitis and edema of the affected limb. These more severe cases generally present with fever, leukocytosis, and hyperglycemia.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree