Lower Extremity Ischemia
Peter K. Henke
James Froehlich
Sanjay Rajagoplan
Gilbert R. Upchurch Jr.
EPIDEMIOLOGY AND USUAL CAUSES
Lower extremity limb pain is a common complaint among patients, particularly the elderly. The first step is to define the cause of the lower extremity pain and, in reference to the topic at hand, to determine whether ischemic arterial vascular disease is the causal factor. Once other common causes of limb pain such as arthritis, low back pain, and musculoskeletal and neurologic causes are eliminated, the workup for ischemic vascular disease should commence. Peripheral arterial disease (PAD) is by far the most common disease manifestation of systemic atherosclerosis in patients, apart from coronary heart disease, and is estimated to occur in up to 15% of persons older than 55 years (1). Risk factors for PAD are the same as those for coronary heart disease and include increasing age, tobacco use, hypertension, hyperlipidemia, male gender, hyperhomocystinemia, and diabetes. Other, less common causes of lower extremity vascular occlusive disease symptoms include Buerger disease (primary small-vessel obliterative arteriopathy associated with tobacco use) and systemic arteritides such as Takayasu arteritis.
The pathophysiologic process of ischemic PAD is critical reduction of blood flow secondary to encroachment of the lumen by atherosclerotic plaque. If the vessel lumen cross-sectional area is narrowed by more than 75%, functionally significant stenosis results, as a consequence of the dramatic impact that alterations in diameter can have on flow. This relation is approximated by Poiseuille’s law (2). The degree of vessel stenosis and whether the stenoses or occlusions are in series or parallel are the most important determinants of the severity of the symptoms and presentation. As muscle activity increases (such as with ambulation), tissue oxygen demand increases, which is compensated for by increased cardiac output, local vasodilation, and increased limb blood flow. In a patient with critical limb ischemia, tissue oxygen demand exceeds delivery even at rest, with anaerobic glycolysis and lactate production, which results in the sensation of pain. The most common anatomic location for infrainguinal atherosclerotic occlusive disease is at the adductor canal (Hunter canal) in the distal superior femoral artery (SFA)/proximal popliteal artery, followed by iliac artery lesions. Tibial arterial occlusive vascular disease is more common in diabetic patients and often occurs at a younger age, although the basic pathophysiologic process is thought to be the same.
PRESENTING SYMPTOMS AND SIGNS
Many asymptomatic patients have underlying PAD evident on arteriography. However, because the occlusion/stenoses occur slowly, collateral vessels develop, and muscle units physiologically adapt. Thus ischemic pain is minimized. In the pelvis and lower extremity, the importance of internal iliac and profunda femoris collateral vessels in maintaining lower limb blood flow cannot be overstated. In general, most patients with cardiovascular disease have PAD of the lower extremities, but whether it should be addressed beyond general risk-factor modification and
exercise depends on the signs and symptoms (of which a full spectrum exists). A detailed and useful set of guidelines for reporting the degree of lower extremity ischemia has been published (3).
exercise depends on the signs and symptoms (of which a full spectrum exists). A detailed and useful set of guidelines for reporting the degree of lower extremity ischemia has been published (3).
The most common and least limb-threatening condition is claudication. This is described typically as limb pain, a sensation of heaviness, or numbness that occurs with ambulation, usually for a reproducibly defined distance, and is relieved by rest. It is important to mention the term disabling claudication, because this is the most subjective indication for an invasive intervention. The decision to perform an intervention should not be done without critical assessment of the patient’s symptoms and living situation. The degree of lower extremity ischemic pain that a patient tolerates is individualized and often dependent on age and occupation. Thus two-block claudication for a sedentary person would not generally warrant intervention. Conversely, a person who ambulates several miles a day for a living may be significantly impaired by the same degree of claudication. The opposite end of the spectrum is rest pain, described as persistent unremitting pain that occurs without any definite preceding lower limb activity. This often occurs at night when the patient is recumbent, and the cardiac output is decreased. The pain is often relieved by limb dependency. Other physical findings with significant ischemic disease include hair loss, muscle atrophy, and marked rubor in the foot. The patient in whom rest pain is diagnosed certainly needs intervention, not only for relief of pain but also to prevent limb loss.
Ulceration is a common manifestation of PAD and often accompanies severe claudication and rest pain. It is important to distinguish ischemic ulceration from venous ulcers (usually medial malleolar in the setting of chronic edema and hyperpigmentation) and neuropathic ulcers (secondary to degeneration of sensory nerves with resultant abnormal pressure distribution on the feet with ambulation). These two types of ulcers may coexist within one patient. Isolated ischemic ulcers are painful and usually occur distally on the foot and toes. These ulcers may progress to frank tissue gangrene and necessitate an urgent amputation if infection supervenes. From a therapeutic standpoint, tissue loss is a definite indication for an intervention to improve blood flow. An endovascular or surgical intervention should precede the débridement or amputation to maximize the chances of tissue salvage, unless the patient has systemic signs of infection.
Overall, any intervention must be judged according to whether the risk entailed by the procedure is less than the benefit derived from the intervention and judged against the possible outcome of severe lifestyle disability and limb loss without the intervention.
A clinical scenario that mandates a slightly different workup is the “blue toe syndrome”; in the typical presentation, a single toe or several toes have a purplish/black appearance; it is usually unilateral; and the toes are quite painful. The presumed cause is atheroembolism. It is important to treat the pain with analgesics, to make sure that the patient is taking antiplatelet therapy, and to initiate a workup that defines the embolic source. In general, this includes an echocardiogram (surface or transesophageal); abdominal, femoral, and popliteal ultrasonography to evaluate for aneurysmal disease; and then either arch-to-outflow aortography (to evaluate for ulcerative atherosclerotic lesions), or CTA of the thoracic and abdominal-pelvic aorta.
Another important differentiation is whether the patient has chronic or acute lower extremity ischemia. Acute limb-threatening ischemia (ALI) may or may not be related to PAD and represents a true emergency, in which the physician must determine the magnitude of the ischemia to prevent limb loss. Limb-threatening ischemia is suggested by the “six Ps”: limb pulselessness, pain, pallor, paresthesias, paralysis, and poikilothermia. The presentation is usually quite dramatic. The most common cause of ALI is a cardiac thromboembolism; the next most common cause is arterial thrombosis in situ. For example, a left atrial thrombus in a patient with atrial fibrillation or left ventricular
aneurysm after a myocardial infarction are typical sources of lower extremity emboli. Less common embolic sources are aortic arch plaques or cardiac tumors.
aneurysm after a myocardial infarction are typical sources of lower extremity emboli. Less common embolic sources are aortic arch plaques or cardiac tumors.
Another common diagnostic consideration is thrombosis in situ, such as in a patient with severe PAD with a significant collateral branch occlusion or a popliteal artery occlusion in the setting of an undiagnosed popliteal artery aneurysm. History and physical examination alone can often enable the physician to distinguish these two scenarios, and the therapeutic approach that follows is slightly different, as discussed later.
HELPFUL TESTS
The history and physical examination of any patient with cardiovascular disease should include evaluation for peripheral manifestations of atherosclerotic occlusive vascular disease. Thorough inquiries about claudication, rest pain, stroke, and neurologic and cardiac symptoms should be obtained. On physical examination, particular attention should be paid to all pulses, both in character and in quality. Loss of hair, shiny dry skin, and trophic nail changes should also be looked for and described. Careful evaluation for any evidence of foot ulcers or deep cracks in the skin between the toes should be noted as well. A physician should be able to determine whether the lower extremity arterial occlusive disease is primarily inflow (e.g., aortoiliac, above the inguinal ligament), outflow (e.g., common femoral artery and distally, below the inguinal ligament), or both, primarily on the basis of the presence or absence of femoral pulses.
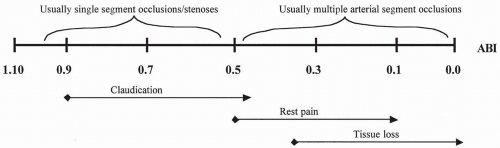 FIGURE 33.1. Schematic of generalized ankle/brachial index, symptoms, and anatomic interrelation in patients with peripheral arterial vascular occlusive disease. |
Once the history and physical examination suggest the presence of PAD, the patient’s segmental limb pressures/Doppler waveforms and ankle/brachial indices (ABIs) should be measured. The ABI is based on the differential blood pressure between the highest brachial systolic pressure and the highest ankle systolic pressure, and indeed, an ABI less than 0.9 is an accepted objective definition of PAD (4). Much practical and important information is obtained through these simple tests for estimating the anatomic level and magnitude of arterial insufficiency. They also allow serial assessment of the patient for progression of disease. Typical ranges of ABI that correlate with symptoms are shown in Fig. 33.1. Full lower extremity duplex arterial examination is not routinely performed at our institution because it is quite time consuming and has not replaced arteriography as the “gold standard” for determining further intervention, although some institutions use this fully. Again, the most important determinant for intervention is severity of patient’s symptoms or the presence of tissue loss in the setting of PAD. The absolute ABI values are not to be used solely as a basis for any intervention, and arteriography, including computed tomographic arteriography, is discouraged as a screening test. However, patients with an absolute toe pressure of less than 40 mm Hg do have an increased risk of tissue loss and may benefit from an intervention, in
comparison with patients with higher pressures but similar claudication symptoms (5). The recommended diagnostic algorithm is shown in Fig. 33.2.
comparison with patients with higher pressures but similar claudication symptoms (5). The recommended diagnostic algorithm is shown in Fig. 33.2.
Two situations bear further discussion: noninvasive testing in diabetic patients and in patients with classic ischemic vascular symptoms but nearly normal ABIs. Diabetic patients have a propensity for arterial medial calcification, which results in noncompressible arteries and thus, invalid ABIs. A toe/brachial index may be a suitable substitute instead, as well as a review of the Doppler waveform pattern. Some surgeons opt directly for arteriography in diabetic patients with tissue loss and without palpable pulses. In patients with suspected significant PAD but normal ABIs, exercise ABIs may be useful for unmasking and confirming a significant occlusive lesion A baseline ABI is measured, and the patient is then put on a treadmill for 5 minutes of ambulation. The ABIs are obtained every minute thereafter to determine the magnitude of pressure decrease
and recovery duration. Both of these are proportional to the degree of stenosis and can help clarify an ischemic cause from other causes of limb pain when the history, physical examination, and resting ABIs do not fully correlate.
and recovery duration. Both of these are proportional to the degree of stenosis and can help clarify an ischemic cause from other causes of limb pain when the history, physical examination, and resting ABIs do not fully correlate.
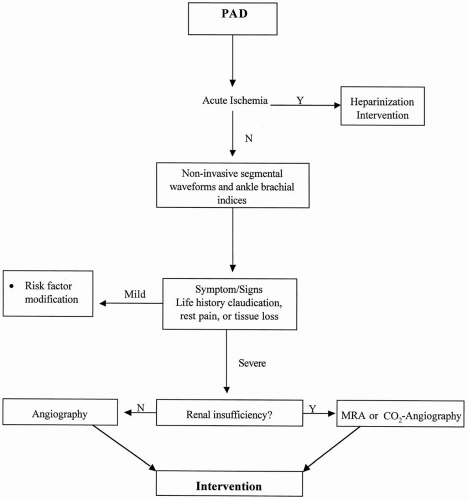 FIGURE 33.2. Suggested algorithm for diagnostic evaluation of patients with peripheral arterial disease. |
Arteriography remains the “gold standard” for determining the anatomic site, severity, and extent of atherosclerotic occlusive disease, although CTA is approaching it for severity and specifically for larger arterial beds.(5a)
It must again be emphasized that patients with stable claudication but without tissue loss should not undergo invasive testing unless interventions are planned, because a definite risk of complications exists. The angiographic images are most often performed in the angiography suite with digital subtraction angiographic techniques. These techniques produce high-resolution images and minimize contrast volume. Some institutions have adopted intraoperative angiography, which is immediately followed by an operative procedure, if indicated (6).
The standard aorta and outflow arteriogram is used to examine the infrarenal aorta, including the renal arteries and oblique views of the pelvis and groin, to define the internal/external iliac and deep/superficial femoral artery bifurcations, respectively. Contrast runoff is performed to assess popliteal and tibial vessels. Foot films are very important for defining suitable targets for a very distal bypass. Of note, in the patient with chronic renal insufficiency in whom contrast-induced nephropathy is a significant risk, gadolinium can be used with good resolution and less risk of impairment of renal function (7). In addition, preprocedural administration of acetylcysteine may decrease the incidence of nephrotoxicity as well as bicarbonate infusion and with preoperative bicarbonate infusion (8).
Magnetic resonance angiography (MRA) has emerged as a useful test for arterial anatomy with very good results. In comparison trials with conventional angiography, sensitivity and specificity for MRA was found to be essentially equivalent with arteriography (9,10). Advantages include less contrast-induced nephropathy risk and the noninvasive nature of the procedure. Furthermore, tissue abnormalities can be determined at the same time, and in at least one study, greater sensitivity for very distal run-off vessels was observed (11). However, the specialized magnetic resonance expertise is not widely available at many hospitals and thus has not replaced arteriography as the “gold standard.”
THERAPY
All patients with PAD require medical and risk factor-reduction therapy regardless of whether they will undergo an invasive procedure. Indeed, a comprehensive consensus statement regarding the diagnosis and treatment has been published (4). The overwhelming priority in the treatment of the patient with PAD is the correction of underlying risk factors that not only may contribute to the progression of disease but also may increase the risk of dying of cardiovascular causes. Of equal importance are reassuring the patient that exercise is excellent therapy for claudication and instituting antiplatelet, hypertension control, and an HMG-CoA reduction inhibitor. Therapy for overall cardiovascular protection is important. Only a minority of patients require some intervention, in the form of pharmacotherapy, surgery, or angioplasty.
Risk Factor Modification/Medical Therapy
Exercise rehabilitation
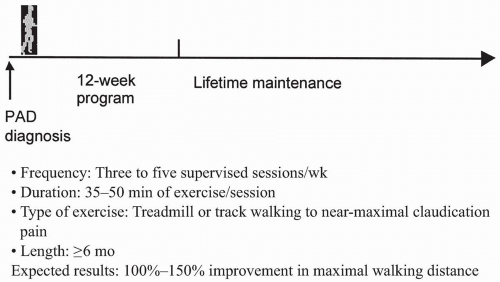
Unequivocal evidence indicates that exercise rehabilitation reduces symptom severity and prolongs claudication distance substantially. A meta-analysis of several prospective controlled studies indicates that the maximal walking distance increases by more than 100% (two blocks or more) (12). The predictors of response appear to be supervised training, high levels of claudication pain during the rehabilitation session, and at least 3 months or more of training. Treadmill exercise appears to be far more effective than
strength training. Figure 33.3 provides some guidelines for initiation of exercise therapy in the patient with claudication.
strength training. Figure 33.3 provides some guidelines for initiation of exercise therapy in the patient with claudication.
Smoking cessation
All tobacco-using patients with claudication should be referred to a smoking-cessation program. For those unable to quit, the use of nicotine replacement therapies in the form of gum, spray, or patch may be considered, with intensive counseling. The various nicotine-replacement therapies significantly decrease symptoms of the withdrawal syndrome as smokers abruptly stop smoking. The different formulations of these therapies provide alternative methods for delivery and have slightly different onsets of action and durations. In meta-analyses, cessation rates with transdermal nicotine range from 15% to 31%, with a trend toward decreased efficacy in the most highly dependent smokers. Nicotine gum studies demonstrate a similar range of cessation rates; the greatest efficacy is seen with the 4-mg gum in highly dependent smokers. Nasal spray cessation rates range from 26% to 28%, also with greatest efficacy in the most dependent smokers (13). Limited inhaler studies report cessation rates similar to those for the nasal spray. Bupropion was initially developed and marketed as an antidepressant medication (Wellbutrin). Although bupropion also aids in smoking cessation, the mechanism by which it does so is unknown. The recommended dosage schedule includes a starting dose of 150 mg per day for 3 days, then increasing to twice per day, with an approximately 25% efficacy rate for tobacco-use cessation. In one published clinical trial (14), “treatment with sustained-release bupropion alone or in combination with a nicotine patch resulted in significantly higher long-term rates of smoking cessation than use of either the nicotine patch alone or placebo.” Abstinence rates were higher with combination therapy than with bupropion alone, but the difference was not statistically significant.
Weight loss
It is generally believed that obesity may contribute to reduction in claudication distance, and weight loss may alleviate this reduction. All obese patients with symptomatic claudication should be encouraged to lose weight. More important, obesity contributes to the risk of hypertension, dyslipidemia, and metabolic syndrome, as well as frank diabetes. As an important risk-factor modification, weight
loss is an essential part of medical therapy for claudication.
loss is an essential part of medical therapy for claudication.
Glycemic control
A strong correlation exists between duration of diabetes and risk of claudication and chronic critical limb ischemia. However, the data on strict diabetes control and amelioration of symptoms of claudication are conflicting. The United Kingdom Prospective Diabetes Study (UKPDS) examined a variety of end points, including peripheral vascular complications with aggressive glycemic control, by using a variety of measures including insulin, sulfonylureas, and metformin in patients with type II diabetes. Tight glycemic control in the study was not associated with improvement in risk for macrovascular events, including peripheral vascular events (15). Because patients with peripheral vascular disease are at risk for the development of foot complications, they should be advised to inspect their feet regularly, avoid pressure points with specially designed footwear, and pay immediate attention to minor cracks and fissures in the skin.
Treatment of hyperlipidemia
Lipid-lowering therapy is among the most effective interventions for the reduction of mortality and morbidity from cardiovascular disease of all kinds. This applies to patients with PAD, too. The greatest risk to life for PAD patients is the risk of heart attack and stroke, which are both reduced substantially by lipid-lowering therapy. The best studied is the use of HMG-CoA reductase inhibitors or statins. The study most pertinent for PAD patients, specifically, is the Heart Protection Study. This study showed that patients with any known cardiovascuclar disease, included those in whom PAD is their only known manifestation of disease, had a significant reduction in death and MI with the use of pravastatin (16). For PAD patients specifically, a 24% reduction in cardiovascular events occurred (17).
For the patient with peripheral arterial disease (PAD), the National Cholesterol Education Program guidelines for the patient with established coronary artery disease are applicable. For these high-risk patients, low-density lipoprotein cholesterol levels above 100 mg per deciliter should be treated aggressively with statins. The goal of therapy should be an LDL level of less than 100 mg per deciliter, with strong consideration given to even lower LDL levels, such as LDL less than 70 mg per deciliter in those with severe or premature disease (18).
As for treatment of patients with high-density lipoprotein (HDL) levels, both the efficacy data and treatment options are currently limited. Exercise and moderate alcohol consumption have been associated with higher HDL levels, and exercise is clearly of benefit in patients with all forms of cardiovascular disease. It is not clear that the benefit of exercise is related to HDL level, however. Data from the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT) suggest that therapy with gemfibrozil can increase HDL levels and is associated with improved cardiovascular prognosis (19). This is especially relevant to the diabetic patient population, whose metabolic profile often comprises low levels of high-density lipoprotein and elevated triglyceride levels (20). These patients would benefit from fibrate therapy.
Hypertension control
Hypertension is a very strong risk factor for the development of all forms of cardiovascular disease, and PAD is no exception. Although no data link blood pressure control to improvements in the natural history of PAD, the overall cardiovascular protective effects are so overwhelming that hypertension control is of great importance. (Due consideration should also be given to a search for secondary causes of hypertension, especially renal artery stenosis.) According to the UKPDS data and the Hypertension Optimal Treatment (HOT) study results, control of blood pressure appears to be far more important than tight glycemic control in diabetic patients (15).
The choices for antihypertensive therapies should be guided by Joint National Committee VII guidelines (21). In this regard, it must be emphasized that no evidence
indicates that β-blockers adversely affect mild to moderate claudication, and they should be considered strongly, especially for the patient with concomitant coronary artery disease. For diabetic hypertensive patients, angiotensin-converting enzyme inhibitors should be the first choice, because of the renoprotective effects of these medications in diabetic patients. On the basis of the benefit of angiotensin-converting enzyme inhibitors in patients with established atherosclerosis, these drugs may be preferred over calcium channel blockers in the initial therapy for uncomplicated hypertension in the patient with PAD (22).
indicates that β-blockers adversely affect mild to moderate claudication, and they should be considered strongly, especially for the patient with concomitant coronary artery disease. For diabetic hypertensive patients, angiotensin-converting enzyme inhibitors should be the first choice, because of the renoprotective effects of these medications in diabetic patients. On the basis of the benefit of angiotensin-converting enzyme inhibitors in patients with established atherosclerosis, these drugs may be preferred over calcium channel blockers in the initial therapy for uncomplicated hypertension in the patient with PAD (22).
Correction of hyperhomocystinemia
Although hyperhomocystinemia is a strong risk factor for PAD, the correction of elevated homocysteine levels with B vitamins and folic acid has not proved to be of clinical benefit. Several randomized trials have tested the use of B vitamins for the treatment of cardiovascular disease. Most recent studies have shown no benefit for the duration of the trials (23,24,25).
Pharmacotherapy for Claudication
Pharmacotherapy for claudication is not meant to replace risk-factor modification or exercise rehabilitation but rather to complement it. The drugs that are currently in use are mentioned as follows.
TABLE 33.1. Drugs used in treatment of claudication | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree