37 Lower Extremity Interventions
 Introduction
Introduction
Peripheral arterial disease (PAD) of the lower extremity is a common health problem. Epidemiological studies have mostly used intermittent claudication (IC) as a symptomatic marker of the disease and an abnormal ankle-brachial index (ABI) to define the burden of asymptomatic PAD. The prevalence of the disease is dependent on the age of the population studied and the underlying atherosclerotic risk profile of the cohort. It is estimated that the overall disease prevalence is in the range of 3% to 10%, increasing to about 15% to 20% in persons older than 70 years. More than half of all patients with lower extremity PAD may be asymptomatic.1–7 History (including standardized questionnaires such as the Rose claudication questionnaire used in the Framingham Heart Study) and physical examination may grossly underestimate the true burden of lower extremity PAD.1 Criqui et al. evaluated the prevalence of lower extremity PAD in a population of 613 men and women in southern California utilizing four different modalities (the Rose questionnaire, pulse examination, ABI, and pulse-wave velocity). The detection rate of PAD with ABI and pulse-wave velocity was 2 to 7 times higher than the detection rate of the Rose questionnaire. Of interest, clinical examination of the pulse overestimated the prevalence of PAD by twofold.8 To optimally manage patients with lower extremity PAD, whether symptomatic or asymptomatic, it is important to understand the global vascular disease burden, the natural history of the disease process, its impact on the patient’s lifestyle, and the risk factors for an individual patient. Such knowledge is key to reducing the mortality and morbidity of the individual patient.
 Vascular Anatomy of the Lower Extremity
Vascular Anatomy of the Lower Extremity
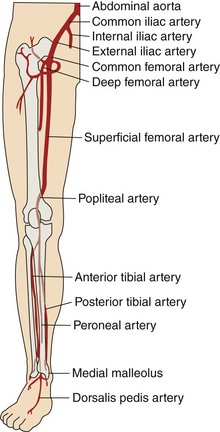
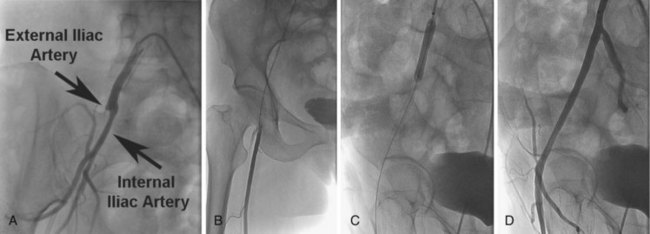
The abdominal aorta bifurcates at the level of the fourth lumbar vertebra into two branches, the right and the left common iliac arteries. The common iliac arteries divide into the external iliac artery, which normally follows the same axis of the common iliac artery, and the internal iliac arteries, which take a posteromedial track in relation to the common iliac artery. In addition to its terminal branches, the common iliac artery gives off branches to the surrounding tissues, peritoneum, psoas muscle, ureter, and nerves. Occasionally, the common iliac artery provides accessory renal arteries to a normal or ectopic kidney. Figure 37-1 depicts the arterial circulation of the lower extremity. The external iliac arteries are larger than the internal iliac arteries. They descend along the medial border of the psoas major muscle, entering the thigh posterior to the inguinal ligament and becoming the common femoral arteries. The inferior epigastric artery arises medially from the distal external iliac artery and ascends behind the rectus abdominis muscle. This vessel is a useful landmark in predicting higher bleeding risk in arterial punctures proximal to its origin.9 The external iliac artery gives off other branches; namely the deep circumflex, cremasteric, and several muscular and cutaneous branches, before it continues as the common femoral artery. Together the common iliac and external iliac arteries contribute to the inflow of the lower extremity. The common femoral artery starts as continuation of the external iliac artery, giving off multiple branches to surrounding tissues, such as the pudendal arteries and the superficial circumflex artery. Then, after giving rise to the arteria profunda femoris (roughly 3.5 cm distal to the inguinal ligament), it becomes the superficial femoral artery (SFA). The profunda femoris arises laterally and posteriorly from the common femoral artery while the SFA continues its pathway to end as the popliteal artery, when it passes through the abductor canal. The profunda gives off perforating branches (usually three, with the end of the profunda as the fourth perforating branch), the circumflex (lateral and medial) arteries, and muscular branches. The popliteal artery continues through the abductor’s canal and, after giving off muscular, cutaneous, genicular and sural branches, terminates in the anterior tibial artery and the tibioperoneal trunk. The common femoral artery, the SFA, the profunda femoris, and the popliteal artery comprise the outflow of the lower extremity. The anterior tibial artery runs between the two heads of the tibialis posterior muscle; then it passes through the upper part of the interosseous membrane to the front of the leg, medial to the head of the fibula. It descends down to the ankle and then continues to the dorsum of the foot, where it becomes the dorsalis pedis artery. The posterior tibial artery arises from the popliteal artery distal to the origin of the anterior tibial artery as the tibioperoneal trunk. After giving rise to the peroneal artery, the tibioperoneal trunk continues as the posterior tibial artery behind the leg and passes behind the medial malleolus. It ends by giving rise to the arteries of the foot, namely the calcaneal, which anastomoses with the calcaneal and malleolar branches of the peroneal, and the medial and lateral plantar arteries. The anterior tibial artery, posterior tibial artery, and the peroneal artery are considered the runoff vessels.
 Diagnosis of Lower Extremity Peripheral Artery Disease
Diagnosis of Lower Extremity Peripheral Artery Disease
History and Physical Examination
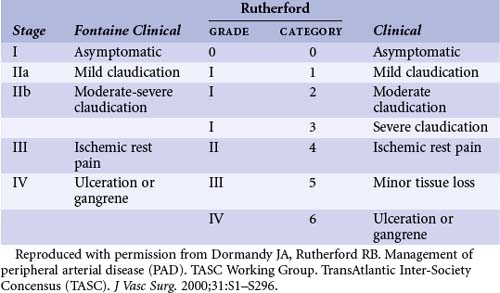
Patients with lower extremity PAD may be asymptomatic (detected during physical examination or screening tests) or may present with IC, rest pain, nonhealing ulcers, or intractable foot infections. In light of the limitations of clinical assessment in defining patients with asymptomatic PAD, additional assessment of patients with risk factors for atherosclerosis may be required in identifying those with asymptomatic advanced PAD. Symptomatic patients can be classified according to the severity of ischemia as based on either the Fontaine or Rutherford categories (Table 37-1).
TABLE 37-1 Classification of Lower Extremity Peripheral Arterial Disease by Fontaine’s Stages and Rutherford’s Categories
Ankle-Brachial Index
Ankle-brachial index (ABI) is a ratio of the blood pressure in the dorsalis pedis or the posterior tibial artery (whichever is higher) to the blood pressure in the brachial arteries. ABI measurement is one of the most cost-effective methods in assessing PAD of the lower extremity and typically is done with a handheld Doppler ultrasound device. This is a noninvasive, fairly reproducible, inexpensive test. The most widely accepted definition of lower extremity PAD is a resting ABI of <0.9. This ABI is usually associated with a 50% or greater angiographic arterial stenosis with a reported sensitivity of 95% and close to 100% specificity.7 A resting ABI of 0.4 to 0.9 is suggestive of mild to moderate PAD and an ABI of <0.4 is suggestive of severe PAD (Table 37-2). Resting ABI measurement can be artifactually high in the setting of the calcification of the tibial arteries that is usually seen in diabetic patients. In that setting the use of toe brachial index (TBI) may be used. A TBI of <0.7 is considered abnormal. The use of exercise ABI may be helpful in equivocal cases. For this the patient walks on the treadmill at a constant speed of 1 to 2 miles per hour at a 10% to 12% incline for 5 minutes, or the exercise can be done with active pedal plantarflexion. A decrease of at least 15 mm Hg in the ankle systolic pressure following the exercise challenge is considered an abnormal test. Patients with no significant PAD are expected to have an increase or no change in their ankle systolic pressure.
TABLE 37-2 Grading Lower Extremity PAD by ABI*
| Supine Resting ABI | Post-exercise ABI | |
|---|---|---|
| Normal | >1.0 | >1.0 |
| Mild | 0.8–0.9 | >0.4 |
| Moderate | 0.4–0.8 | >0.2 |
| Severe | <0.4 | <0.2 |
* Post exercise ABIs are measured following treadmill exercise at 1 to 2 mph, 10% to 12% grade, for 5 minutes or symptom-limited.
Reprinted with permission Mukherjee D, Yadav JS. Update on peripheral vascular diseases: from smoking cessation to stenting. Cleve Clin J Med. 2001;68(8):723–733.
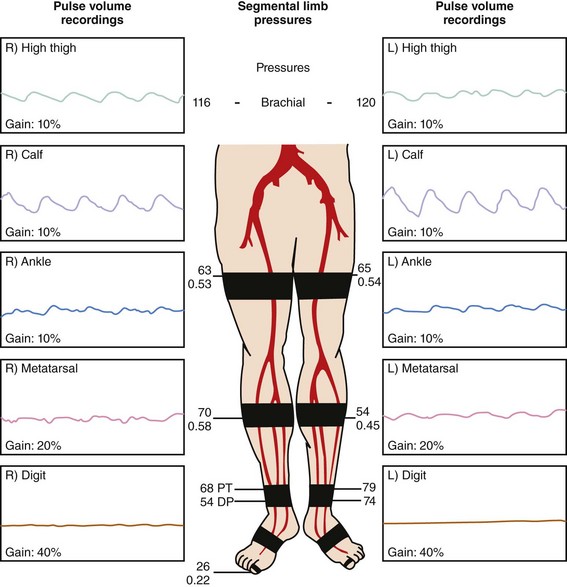
Pulse Volume Recording
Plethysmography is used to detect volumetric changes in blood flow to the lower extremity; it is performed at various segments of the lower extremity with pressure cuffs inflated to 60 to 65 mm Hg. Normal tracing will have a rapid systolic upstroke and downstroke with a prominent dicrotic notch. This pattern changes as PAD develops and progresses, with a noted attenuation and widening of the arterial waveform. Ultimately, the waveform becomes flat (nonpulsatile) in patients with advanced PAD (Fig. 37-2).
Computed Tomography Angiography
The use of spiral CT angiography (CTA) in assessing lower extremity PAD has 93% sensitivity and 96% specificity in detecting >50% stenosis with high accuracy when compared with digital subtraction angiography. A recent systematic review and metanalysis suggested that CTA is highly accurate for assessment of PAD in all regions of the lower extremity arteries.10 CTA correctly identified hemodynamically significant lesions and also accurately distinguished between >50% stenoses and occlusions. Some 94% of occlusions and 87% of nonoccluded segments with more than 50% stenosis detected by contrast angiography were correctly identified by CTA.10 The accuracy of CTA may actually be even higher than reported in this study because all studies used contrast angiography as the reference standard but did not report whether biplanar views were used routinely. Because CTA reconstructions allow three-dimensional assessment, significant disease may be detected by CTA but not be recognized by angiography, which may lead to underestimation of specificity. CTA has advantages over magnetic resonance angiography (MRA) in the case of patients with pacemakers and defibrillators and in those with metal clips, stents, or prostheses (no significant artifact is seen in CTA as opposed to MRA) and is significantly faster to perform as compared with MRA. However, CTA requires the use of iodinated contrast and entails exposure to ionizing radiation. Dose-saving algorithms are very effective in reducing radiation exposure and should be used whenever possible.11
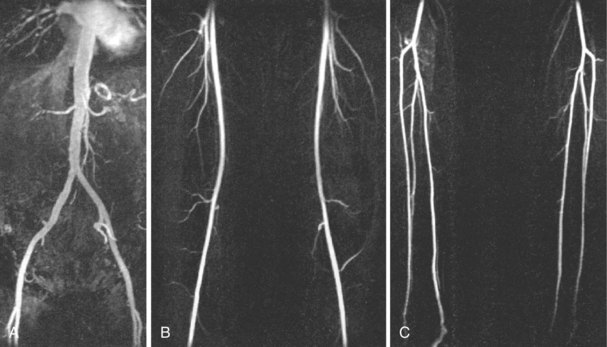
Magnetic Resonance Angiography
The use of gadolinium-enhanced MR angiography (GEMRA) (Fig. 37-3) in the assessment of lower extremity PAD has been compared with standard catheter angiography with a reported sensitivity and specificity of about 90% and 100%, respectively, for detecting stenosis >50%. Most contemporary studies report an agreement of 91% to 97% between MRA and catheter angiography. GEMRA is superior to duplex ultrasound in detecting >50% stenotic lesions (sensitivity of 98% vs. 88% and specificity of 96% vs. 95%).7 Limitations of this technology include the tendency to overestimate the severity of stenosis, metal clips may give the impression of total occlusion, and metal stents can also obscure vascular flow. In addition to these limitations, there are certain subset of patients who cannot be studied with MRA, such as those with pacemakers and defibrillators and those with certain types of cerebral aneurysm clips.7 The principal role of MRA is in the initial evaluation for PAD, especially in patients with inflow disease, using “bolus chase” three-dimensional imaging, where a single bolus of contrast is followed to the foot.
Contrast Angiography
Despite recent advances in the noninvasive evaluation of lower extremity PAD, contrast angiography remains the gold standard. Traditionally, a pelvic/abdominal aortogram in the anteroposterior projection is done using a straight pigtail catheter (5 or 6 Fr) placed at the level of the L1-L2 vertebrae. Approximately 10 to 15 mL of iso-osmolar contrast is injected at a rate of 15 mL/s with digital subtraction angiography (DSA) technology. This provides an excellent view of the distal aorta and the origin of the common iliac arteries and the external iliac and common femoral arteries. Angulated views (LAO of 30 degrees) can then be used to visualize the iliac and femoral bifurcations without overlap. Then the pigtail catheter is placed above the aortic bifurcation (L3-L4) and digital subtraction with bolus chase, 8 mL/s for 10 seconds, is used to assess the outflow and distal runoff. Selective injections and sheath injections can then be used as needed to further define the territory of interest. “Roadmap” technology can be employed subsequently to help operators in their intervention and the placement of balloons and stents. Contrast angiography remains the most easily used and widely available imaging technique in patients with PAD of the lower extremity when revascularization is contemplated. Noninvasive technologies, such as MRA or CTA, may be used prior to contrast angiography to help identify the potential culprit lesion and plan the best approach to study such lesion (access point, catheter selection, etc.) invasively.7 Contrast angiography may be associated with vascular access complications (bleeding, infections, pseudoaneurysms, and vascular disruption), atheroembolism, and contrast nephropathy as well as contrast-induced anaphylactoid reactions. These complications, although rare, should be considered in the decision-making process with regard to the assessment of PAD.
 Interventions
Interventions
Iliac Artery Intervention (Inflow Disease)
Revascularization Options
Occlusive disease confined to the iliac arteries appears to occur in relatively young patients and may therefore have a greater impact on productivity and lifestyle. For instance, the mean age of the cohort in the Dutch Iliac Stent Trial was approximately 59 years.12 These patients (>90% were smokers) were otherwise healthy compared with those with infrainguinal disease or more diffuse PAD. In general, any type of revascularization for this subset of patients can offer satisfactory long-term results. Historically, aortobifemoral bypass surgery has been the gold standard for PAD involving the iliac arteries, as this procedure is associated with excellent long-term patency rates (85%–90% at 5 years, 75%–80% at 10 years, and 60% at 20 years); however, it may be associated with an intraoperative mortality of roughly 1% to 3%, and a major complication rate of 5% to 10%. This, combined with the excellent intermediate to long-term patency rates following percutaneous intervention, has led to the emergence of percutaneous revascularization as an attractive alternative to surgery in patients with suitable lesions for such intervention. In the Swedish randomized controlled trial, where 37% of randomized patients had iliac artery stenosis, there was equivalence in outcomes between percutaneous transluminal angioplasty (PTA) and surgery.13 In the iliac disease subgroup, the patency rate at one year was 90% in the PTA arm versus 94% in the surgical arm. Table 37-3 summarizes the strategy recommended by the Trans-Atlantic Inter-Society Consensus (TASC) working group for the revascularization of iliac lesions.
TABLE 37-3 Recommendations of the TransAtlantic Inter-Society Consensus (TASC) Working Group for the Revascularization Strategy of AortoIliac Femoropopliteal Lesions*
| Type | Iliac Disease | Femoral Lesions |
|---|---|---|
| TASC A | Single <3 cm of CIA or EIA (unilateral/bilateral) | Single ≤3 cm in length, not involving SFA or popliteal |
| TASC B | ||
| TASC C | ||
| TASC D | Complete CFA or SFA occlusions |
CIA, Common iliac artery; EIA, external iliac artery; SFA, superficial femoral artery; CFA, common femoral artery; AAA, abdominal aortic aneurysm.
* Endovascular procedure is the treatment of choice for type A; surgery is the procedure of choice for type D. At present, endovascular treatment is more commonly used in type B lesions, and surgical treatment is more commonly used in type C lesions.
Reproduced with permission from Dormandy JA and Rutherford RB, Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Concensus (TASC). J Vasc Surg. 2000;31:S1–S296.
 Techniques
Techniques
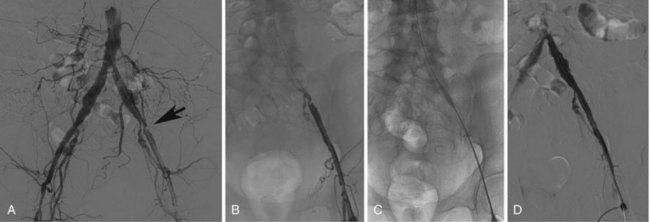
Access and Recanalization Techniques
In patients with unilateral stenotic iliac lesions that do not involve the common femoral artery (CFA), ipsilateral access through the CFA with retrograde PTA is usually preferred as it provides direct access to the diseased segment as well as a coaxial alignment of the equipment (Fig. 37-4A to D). Contralateral access with the use of a crossover sheath is reserved for patients with disease involving the ipsilateral CFA (Fig. 37-5A to D), if there are plans to intervene on more distal lesions in the same limb, or if there is concern about jeopardizing the flow to the affected limb by placement of the sheath in the ipsilateral CFA. Various crossover sheaths are available; the ArrowFlex (Arrow International, Reading, PA) sheath is a more flexible sheath, which can be helpful in crossing over acute aortic bifurcation angles but provides less support as opposed to the RAABE and Balkin sheaths (both from Cook Inc., Bloomington, IN), which provide more support but are less compliant. If the iliac artery is occluded, either or both approaches may be needed. For lesions of the aortic bifurcation, the optimal approach is a bilateral retrograde one with the placement of kissing balloons and subsequently kissing stents.
Clinical Data
Percutaneous revascularization in the management of patients with IC secondary to iliofemoral disease (specifically PTA without stenting) has been compared with conservative management (specifically with exercise training, smoking cessation counseling, and antiplatelet therapy with aspirin). The results revealed two important findings: first that PTA can effectively alleviate patients’ symptoms and improve treadmill distance and ABI during a short-term follow-up period, but these benefits are mostly lost by 2 years. The second observation was that although PTA improves perfusion to the feet, which may confer protection particularly for populations at higher risk for limb loss, such as diabetics, supervised exercise improves functional outcome and also enhances global conditioning. These two strategies should therefore be considered complementary because they address different but interrelated issues.14–16 Overall, for iliofemoral lesions, the clinical results of percutaneous revascularization are generally comparable with those of surgical bypass or reconstruction. The Swedish trial randomized patients with threatened limb loss (40% with rest pain or gangrene) or claudication who had not improved with exercise training (60%) to either PTA or surgical revascularization.17
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree