Williams syndrome (WS) is a congenital disorder affecting the vascular, connective tissue, and central nervous systems of 1 in 8,000 live births. Previous reports have reported high frequencies of cardiovascular abnormalities (CVAs) in small numbers of patients with WS. A retrospective review was undertaken of patients with WS evaluated at our institution from January 1, 1980 through December 31, 2007. WS was diagnosed by an experienced medical geneticist and/or by fluorescence in situ hybridization. CVAs were diagnosed using echocardiography, cardiac catheterization, or computed tomographic angiography. Freedom from intervention was determined using Kaplan-Meier analysis. The study group was 270 patients with WS. The age at presentation was 3.3 ± 5.9 years with follow-up of 8.9 ± 9.0 years (range 0 to 56.9). CVAs were present in 82% of the patients. The most common lesions were supravalvar aortic stenosis in 45% and peripheral pulmonary stenosis in 37%; 20% had both. Other common lesions included mitral valve prolapse and regurgitation in 15%, ventricular septal defect in 13%, and supravalvar pulmonary stenosis in 12%. Surgical or catheter-based interventions were performed in 21%. The rate of freedom from intervention was 91%, 81%, 78%, 72%, and 62% at 1, 5, 10, 20, and 40 years. Eight patients died. In conclusion, CVAs are common in patients with WS, but supravalvar aortic stenosis and peripheral pulmonary stenosis occurred less frequently in this large cohort than previously reported. In patients with WS and CVAs, interventions are common and usually occur by 5 years of age. Most patients with WS do not require intervention during long-term follow-up, and the overall mortality has been low.
Multiple investigators have reported an array of cardiovascular findings in cohorts with Williams syndrome (WS). Most of the available studies describing cardiovascular abnormalities (CVAs) have been small, with sample sizes of 10 to 127 subjects. Pham et al have reported on 242 patients ranging in age from 1 day to 22.7 years, without a delineation of age at presentation. These patients required cardiac catheterization and/or surgery at 47 cardiac centers. In 75 patients, Eronen et al have demonstrated that interventions are more frequently needed in infants with WS than in children or adults with WS. Studies delineating the long-term outcomes as a function of CVA severity have not been performed. The paucity of information describing CVAs and outcomes in those with WS has limited the clinician’s ability to care for patients and give guidance to parents. The aims of the present study were to (1) assess the prevalence and types of CVAs in a large cohort of patients with WS, and (2) characterize the outcomes of patients with CVAs in this cohort.
Methods
We retrospectively reviewed the available charts of patients with the diagnosis of WS who were evaluated at the Children’s Hospital of Philadelphia (Philadelphia, Pennsylvania) from January 1, 1980 through December 31, 2007. The available records for this cohort included dates spanning from August 16, 1965 to March 24, 2008. The records were reviewed for all patients seen at the Multidisciplinary Williams Syndrome Clinic and/or the cardiology division. All patients who were initially evaluated by a geneticist were subsequently evaluated by a pediatric cardiologist. The diagnosis of WS was confirmed by the clinical phenotype assessed by an experienced medical geneticist and/or by demonstrating elastin hemizygosity by fluorescence in situ hybridization. Congenital CVAs were diagnosed using echocardiography, cardiac catheterization, or computed tomographic angiography. The hospital’s institutional review board approved the study.
All available cardiovascular data on the identified patients were reviewed. The patient histories, physical characteristics, and ancillary testing, including abdominal ultrasonography and magnetic resonance imaging/magnetic resonance angiography of the brain (evaluating for Chiari I sequence and/or stroke) and renal arteries (in the setting of hypertension or inadequate renal growth), were reviewed. All patients were evaluated with echocardiography and, as clinically indicated, by cardiac catheterization and/or computed tomographic angiography. Cardiac catheterization data, including hemodynamics, angiographic information, and catheter-based interventions (CBIs), were reviewed. The surgical records were analyzed to determine the anatomic measurements and interventions performed.
CVAs were defined as any anatomic or functional abnormality of the heart or vascular system. Patients with either isolated systemic hypertension or the isolated combination of systemic hypertension and trivial mitral regurgitation were not considered to have CVAs. The severity of individual lesions was graded using published standards of echocardiography and/or catheterization. When pressure gradients were available from catheterization or echocardiography, the catheter-derived gradients were determined by peak-to-peak measurements. The echocardiographically derived gradients were determined by peak instantaneous measurements. When both catheter- and echocardiographic-derived pressure gradient data were present, the catheter data were used for the determination of lesion severity. Any lesion graded as trivial was not included in the statistical analysis.
Proportional analysis was used to determine the differences in gender distributions. The Student t test was used to analyze the gender differences in CVAs. Cox regression analysis was used to determine differences between severity grades within individual lesion groups. Kaplan-Meier analysis was used to determine freedom from lesion-specific intervention (ie, peripheral pulmonary stenosis [PPS] intervention for those with PPS and supravalvar aortic stenosis [SVAS] intervention for those with SVAS) for both PPS and SVAS, and freedom from all cardiovascular interventions, independent of lesion type. Freedom from intervention analysis was based on lesion severity at presentation. Statistical significance was considered present when p <0.05.
Results
A total of 270 patients (136 females and 134 males) with WS constituted the study cohort. Of the 270 patients, 221 (82%) had CVAs; 111 were female and 107 were male. The mean age at the initial evaluation by a pediatric cardiologist was 3.3 years (range 0 to 39.0). More than 1/2 of all patients (51%) had presented by 1 year of age. No gender difference was found in the age at initial presentation. The mean length of follow-up was 8.9 years (range 0 to 56.9). The mean age at the diagnosis of WS was 4.9 years (range 0 to 43.0).
Of the CVAs present, SVAS, PPS, mitral valve prolapse and regurgitation, coarctation of the aorta, ventricular septal defect, and supravalvar pulmonary stenosis each had a prevalence of >10%. Other CVAs were seen less frequently. Table 1 lists the frequencies of specific CVAs in these patients.
| Variable | Frequency (n = 270) | Patients With Intervention (n = 56) | Catheter-Based Intervention (n = 17) | Surgical Intervention (n = 48) |
|---|---|---|---|---|
| Stenotic vascular lesions | ||||
| Supravalvar aortic stenosis | 121 (45%) | 34/121 (28%) | 1/34 (3%) | 33/34 (97%) |
| Peripheral pulmonary stenosis | 99 (37%) | 17/34 (50%) | 14/17 (82%) | 8/17 (47%) |
| Aortic coarctation | 37 (14%) | 9/37 (24%) | 3/9 (33%) | 8/9 (89%) |
| Arterial stenosis | 37 (14%) | |||
| Renal | 19 (7%) | 1/18 (6%) | 0 | 1/1 (100%) |
| Neck and limbs | 7 (3%) | 0 | 0 | 0 |
| Abdominal aorta | 5 (2%) | 0 | 0 | 0 |
| Mesenteric | 5 (2%) | 1/4 (25%) | 0 | 1/1 (100%) |
| Intracranial | 3 (1%) | 0 | 0 | 0 |
| Supravalvar pulmonary stenosis | 31 (12%) | 5/31 (16%) | 1/5 (20%) | 4/5 (80%) |
| Coronary anomalies | 16 (6%) | |||
| Ostial stenosis | 14 (5%) | 2/14 (14%) | 0 | 2/2 (100%) |
| Anomalous left coronary arising from | ||||
| Pulmonary artery ⁎ | 1 | 1/1 (100%) | 0 | 1/1 (100%) |
| Circumflex right sinus ⁎ | 1 | 0 | 0 | 0 |
| Cardiac lesions | ||||
| Ventricular septal defect | 34 (13%) | 3/34 (9%) | 0 | 3/3 (100%) |
| Valvar pulmonary stenosis | 23 (9%) | 2/23 (9%) | 1/2 (50%) | 1/2 (50%) |
| Secundum atrial septal defect | 9 (3%) | 1/9 (11%) | 0 | 1/1 (100%) |
| Valvar aortic stenosis | 6 (2%) | 2/6 (33%) | 1/2 (50%) | 1/2 (50%) |
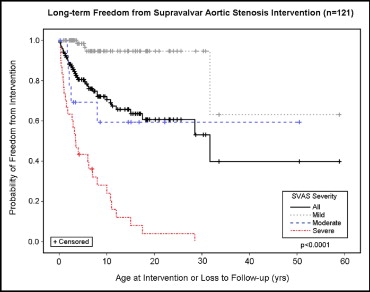
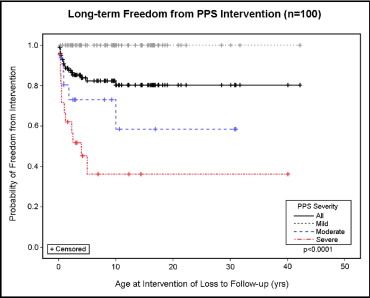
SVAS was the most common CVA in this cohort. Table 2 lists the SVAS lesion severities. Spontaneous improvement was seen in the severity grade of SVAS in 16 (13%) of 121 patients during follow-up, with 12 (16%) of 77 patients in the mild group showing complete resolution. Of the 121 patients with SVAS, 12 (10%) demonstrated progression in the severity of SVAS during follow-up. The differences in the freedom from SVAS-specific intervention were highly significant among the severity groups (p <0.0001; Figure 1 ). Peripheral pulmonary stenosis was the second most common CVA in this cohort ( Table 1 ). The PPS severities are listed in Table 2 . During follow-up, the grade of PPS severity spontaneously improved in 37 (37%) of 100 patients, with 25 (40%) of the 62 in the mild group showing complete resolution. Most patients (68%; 25 of 37) with spontaneous improvement in the severity of PPS had initially had mild stenosis, although improvement to mild stenosis was seen in patients with moderate and severe PPS. Two patients with moderate PPS progressed to severe PPS. The differences in the freedom from PPS-specific intervention were highly significant among the severity groups (p <0.0001; Figure 2 ).
| Variable | Mild | Moderate | Severe |
|---|---|---|---|
| Supravalvar aortic stenosis | 77/121 (64%) | 14/121 (12%) | 30/121 (25%) |
| Peripheral pulmonary stenosis | 62/100 (62%) | 16/100 (16%) | 22/100 (22%) |
| Coarctation of the aorta | 18/37 (49%) | 10/37 (27%) | 9/37 (24%) |
| Supravalvar pulmonary stenosis | 15/31 (48%) | 9/31 (29%) | 7/31 (23%) |

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


