Transcatheter aortic valve implantation (TAVI) is an established technique for the treatment of severe symptomatic aortic stenosis. Data on long-term TAVI outcomes, both hemodynamic and clinical, in real-world practice settings are limited. We aim to explore the long-term clinical results in patients with severe symptomatic aortic stenosis using multiple catheter-based options: 360 TAVI-treated patients were followed up for ≤5 years. The Medtronic CoreValve was used in 71% and the Edwards SAPIEN in 26%. The primary end point was all-cause mortality during follow-up. Outcomes were assessed based on the Valve Academic Research Consortium 2 criteria. The mean ± SD patient age was 82.1 ± 6.9 years (56.4% women). The Society of Thoracic Surgeons score was 7.5 ± 4.7. The clinical efficacy end point and time-related valve safety at 3 years was 50% and 81.7%, respectively. The calculated 3- and 5-year survival rates were 71.6% and 56.4%, respectively. Five-year follow-up data were obtained for 54 patients alive; 96.2% of alive patients were in the New York Heart Association class I and II, 4 years after TAVI. No gender differences in all-cause mortality rates were observed (p = 0.58). In multivariate analysis, hospitalization 6 months previous to TAVI (hazard ratio [HR] 1.92, 95% confidence interval [CI] 1.17 to 3.15, p = 0.01), frailty (HR 1.89, 95% CI 1.11 to 3.2, p = 0.02), acute kidney injury (HR 1.93, 95% CI 1.03 to 3.61, p = 0.04), and moderate or more paravalvular aortic regurgitation after TAVI (HR 4.26, 95% CI 2.54 to 7.15, p <0.001) were independent predictors for all-cause mortality. In conclusion, long-term outcomes of TAVI are encouraging. Prevention and early identification of paravalvular leak and acute renal failure after the procedure would improve short- and long-term outcomes.
Transcatheter aortic valve implantation (TAVI) is a well-established therapeutic option for severe symptomatic aortic stenosis (AS) in patients at high surgical risk and is the treatment of choice in nonsurgical candidates. In the present study, we report our long-term experience in treating patients with AS using the TAVI technique. Patients were followed for up to 5 years. We also assessed potential differences in outcomes based on gender differences.
Methods
From November 2008 to March 2015, 1,003 patients with severe symptomatic AS were referred to a dedicated consultation “heart team” forum and clinic in our medical center. This forum includes a multidisciplinary team of clinical cardiologists, cardiac imaging specialists, interventional cardiologists, cardiac surgeons, and geriatricians. To determine the most appropriate treatment, each patient underwent a rigorous assessment process. Severe AS was defined as a valvular orifice area <1.0 cm 2 or <0.6 cm 2 /m 2 and/or mean pressure gradient >40 mm Hg and/or jet velocity >4.0 m/s.
Selected patients with discordant echocardiographic findings underwent dobutamine echocardiographic stress test and were classified into 3 groups: (1) low-flow, low-gradient, low left ventricular ejection fraction (LVEF%) severe AS (8%), (2) low-flow, low-gradient, preserved LVEF% severe AS (2.2%), and (3) low-flow, low-gradient, low LVEF% without AS.
All patients considered for valve replacement were assessed by coronary angiography, and 88.6% and 45.6% of those selected for TAVI underwent transesophageal echocardiography and gated cardiac computed tomography (CT), respectively, for valve sizing and calcium burden assessment. Vascular access was assessed by multislice CT and peripheral angiography. Both the logistic European System for Cardiac Operative Risk Evaluation score (log-EuroScore) and Society of Thoracic Surgeons (STS) score were calculated. Patients with an estimated life expectancy shorter than 1 year or mental impairment were referred for palliative medical treatment and/or balloon aortic valvuloplasty. Patients who were deemed to be suitable candidates for valve replacement with a low operative risk, defined as a log-EuroScore <15% and/or an STS score <8%, without contraindications for surgery, were assigned to surgical aortic valve replacement. Patients with a log-EuroScore >15% and an STS score >8% were evaluated individually and referred for TAVI. Starting from 2013, patients >75 years, with STS score from 4% to 8% and multiple co-morbidities and/or frailty, were also considered for TAVI treatment by the heart team. The following parameters are considered to define frailty in our practice: gait speed, ability to perform activities of daily living, albumin levels, need for oxygen replacement therapy, cognitive status, patient’s general appearance, and the impression of the treating clinician.
Patients with >6-mm iliac-femoral inner artery diameter were considered for transfemoral procedure. In patients in whom the transfemoral access was inappropriate, the transaxillar, transapical, transaortic, and or retroperitoneal accesses were chosen according to patients’ characteristics.
Our institution used both the Edwards SAPIEN system (Edwards Lifesciences, Irvine, California) and the Medtronic CoreValve prosthesis (Medtronic, Minneapolis, Minnesota) starting from 2008. In 2010, the Edwards SAPIEN XT replaced the Edwards SAPIEN valve. In 2014, the Corevalve Evolut and Corevalve Evolut R (Medtronic), the Lotus Valve System (Boston Scientific Corporation, Marlborough, Massachusetts), and the Edwards SAPIEN 3 (Edwards Lifesciences) were added.
The characteristics favoring one device over the other overlapped in most of the cases. However, the presence of electrical conduction abnormalities and transapical approach favored the implantation of an Edwards device, whereas large aortic valve annulus, transaxillar access, and borderline femoral size favored the implantation of a Corevalve device.
In our practice, TAVI candidates are hospitalized 36 hours before the planned procedure in the clinical cardiology or the geriatrics departments. During this preprocedure hospitalization, patients underwent a comprehensive social, functional, and cognitive assessment. TAVI procedures were performed in our catheterization suite. Initial balloon valvuloplasty was frequently performed during the first 3 years of our TAVI program and abandoned as a routine practice afterward. At present, this is performed only in selected cases. Rapid right ventricular pacing was performed during deployment of the Edwards SAPIEN valve and only occasionally during Medtronic CoreValve implantation. The TAVI procedure was considered successful if a single implanted valve was in the appropriate location and functioning properly in the absence of any major complications, according to the Valve Academic Research Consortium 2 definition.
The institutional review board approved this study (i.e., RECORD TAVI Database). The primary end point of the present study was all-cause mortality after TAVI. The secondary end points were death from cardiovascular causes, changes in the New York Heart Association (NYHA) functional class, the immediate and long-term valve gradients performance of the valve, the presence of residual aortic paravalvular leak (PVL), and stroke after the TAVI procedure. We also report the results of an analysis between the outcomes of patients stratified by gender. All patients were prospectively followed up at 30 days, 6 and 12 months, and yearly thereafter, after TAVI. A 100% follow-up rate was achieved during the assigned period.
All TAVI-related data were registered in an electronic file and analyzed using the SPSS, version 20.0, software (SPSS, Chicago, Illinois). Data are presented as mean ± SD for continuous variables. Continuous variables were compared using the Student’s t test, and categorical variables were compared using the chi-square statistics with the Fisher’s exact test, as appropriate. All tests were 2 tailed, and a p value <0.05 was considered significant. All-cause and cardiac mortality were analyzed using the Kaplan-Meier procedure. Survival analysis stratified by gender comparison was performed using the log-rank test. Univariate and multivariate Cox regression analyses were applied to assess the dependent and independent predictors of all-cause mortality, respectively. The variables included in the present analysis were all clinical, laboratory, and echocardiographic parameters that can affect the prognosis before and after TAVI. The multivariate analysis was performed using the predictors found to be significant on the univariate analysis.
Results
From November 2008 to March 2015, 402 patients were treated with the percutaneous implantation of a valve device in our institution; 41 valve-in-valve implantations were performed for the treatment of bioprosthetic valve deterioration and 1 direct implantation in a native mitral valve. The present report focused on the outcomes of the 360 patients assigned to TAVI for the treatment of severe symptomatic AS. The mean follow-up was 681 ± 441 days. Patient characteristics and echocardiographic findings are listed in Table 1 .
| Variable | Value |
|---|---|
| Age (years) | 82.1 ± 6.9 |
| Female | 203 (56.4%) |
| Body mass index (kg/m 2 ) | 27.8 ± 14.6 |
| Diabetes mellitus type 2 | 122 (34%) |
| Hypertension | 332 (92.2%) |
| Dyslipidemia ∗ | 312 (86.7%) |
| Mean glomerular filtration rate † | 65.6 ± 16.4 |
| Glomerular filtration rate <90 ml/min/1.73 m 2 | 299 (83%) |
| 60-89 | 138 (38.3%) |
| 30-59 | 134 (37.2%) |
| 15-29 | 18 (5%) |
| <15 | 9 (2.5%) |
| Previous stroke or transient ischemic attack | 66 (18.3%) |
| Peripheral vascular disease | 59 (16.4%) |
| Anemia ‡ | 234 (65%) |
| Chronic obstructive lung disease | 76 (21%) |
| “Porcelain” Aorta | 21 (5.8%) |
| Pacemaker | 30 (8.3%) |
| Atrial fibrillation | 113 (31.4%) |
| Previous coronary bypass surgery | 71 (19.7%) |
| Previous coronary angioplasty | 140 (39%) |
| Previous myocardial infarction | 30 (8.3%) |
| Society of Thoracic Surgeons score | 7.5 ± 4.7 |
| European System for Cardiac Operative Risk Evaluation Score | 19.5 ± 11.2 |
| New York Heart Association Functional Class III – IV | 343 (95.3%) |
| Hospitalization in previous 6 months to TAVI | 198 (55%) |
| Peak aortic valve gradient (mmHg) | 78.5 ± 24.4 |
| Mean aortic valve gradient (mmHg) | 49.4 ± 16.8 |
| Mean Aortic Valve Area (cm 2 ) | 0.6 ± 0.2 |
| Coronary and Peripheral Angiography | 356 (99%) |
| Trans-esophageal Echocardiography | 319 (88.6%) |
| Cardiac Computed Tomography | 164 (45.6%) |
| Mean Peak to Peak gradient (mmHg) | 53 ± 21 |
| Left Ventricular Dysfunction >Moderate | 29 (9.9%) |
∗ Dyslipidemia was defined as total cholesterol >200 mg/dl, low-density lipoprotein >130 mg/dl, high-density lipoprotein <40 mg/dl, and/or triglycerides >200 mg/dl.
† Glomerular filtration rate was calculated by the Modification of Diet in Renal Disease formula.
‡ Anemia was defined as hemoglobin <12 mg/dl for women and <13 mg/dl for men.
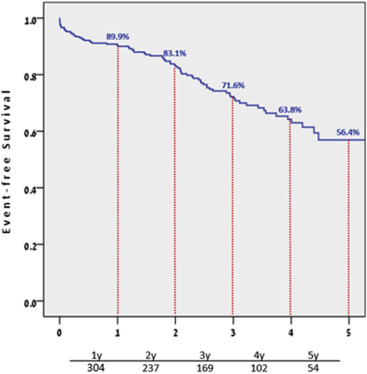
Procedural details and outcomes are presented in Table 2 . Pacemaker implantation after TAVI was required in 58 patients (17.6%) because of high-grade electrical conduction abnormalities: 20% and 4.3% of patients treated with the Medtronic CoreValve and Edwards SAPIEN valve, respectively. Of the 360 patients initially included in our study, 81 died during the 5-year follow-up period. Cardiovascular cause of death, including heart failure and procedure-related events, was noted in 27 patients (33%), the majority of these deaths, 19 (70%) occurred in the first year after the procedure. The calculated 1-, 3- and 5-year survival rates were 89.9%, 71.6%, and 56.4%, respectively. The overall Kaplan-Meier survival curve is depicted in Figure 1 .
| Variable | Value |
|---|---|
| Safety and efficacy end points | |
| Death | |
| At 30 days | 14 (3.9%) |
| From non-cardiac cause | 1 (0.3%) |
| From cardiovascular cause | 13 (3.6%) |
| At 1 year | 31 (8.6%) |
| From non-cardiac cause | 12 (3.3%) |
| From cardiovascular cause | 19 (5.3%) |
| At 2 years | 46 (12.8%) |
| From non-cardiac cause | 23 (6.4%) |
| From cardiovascular cause | 23 (6.4%) |
| At 3 years | 67 (18.6%) |
| From non-cardiac cause | 41 (11.4%) |
| From cardiovascular cause | 26 (7.2%) |
| At 4 years | 76 (21.1%) |
| From non-cardiac cause | 49 (13.6%) |
| From cardiovascular cause | 27 (7.5%) |
| At 5 years | 80 (22.2%) |
| From non-cardiac cause | 53 (14.2%) |
| From cardiovascular cause | 27 (7.5%) |
| Periprocedural Myocardial Infarction | 2 (0.5%) |
| Ischemic Stroke Any at 30 days | |
| Major Ischemic Stroke at 30 days | |
| Minor Ischemic Stroke at 30 days | |
| Life-Threatening Bleeding | 5 (1.4%) |
| Major bleeding | 7 (1.9%) |
| Minor Bleeding | 16 (4.4%) |
| Acute Kidney Injury AKIN Classification Stage I | 43 (11.9%) |
| Acute Kidney Injury AKIN Classification Stage II | 5 (1.4%) |
| Acute Kidney Injury AKIN Classification Stage III | 11 (3%) |
| Vascular Complications Any | 68 (18.9%) |
| Minor Vascular Complication | 61 (16.9%) |
| Major Vascular Complication | 7 (1.9%) |
| Prosthetic Valve Performance | |
| Aortic Stenosis Mild at 30 days | 1 (0.3%) |
| Aortic Stenosis Moderate / Severe at 30 days | 0 (0%) |
| Aortic Stenosis Mild at 4 years | 5 (8.9%) |
| Aortic Stenosis Moderate / Severe at 4 years | 2 (3.5%) |
| Prosthetic Valve Associated Complications | |
| Conduction Disturbances | |
| New onset left bundle branch block | 62 (19.7%) |
| Need for pacemaker implantation | 58 (16.1%) |
| Coronary Obstruction | 2 (0.5%) |
| Clinical benefit end-points | |
| Hospitalization for symptoms of cardiac decompensation | 13 (5.2%) |
| Therapy-specific endpoints | |
| Conversion to open surgery | 3 (0.8%) |
| Ventricular perforation with tamponade | 6 (1.7%) |
| Post-dilatation balloon valvuloplasty | 65 (18.1%) |
| Need for a second valve | 18 (5%) |
| Valve snaring in Corevalve implantations | 10 (3.9%) |
| Composite endpoints | |
| Device success (single valve) | 325 (90.3%) |
| Device success (including a 2nd valve) | 343 (95.3%) |
| Early safety endpoint at 30 days | 268 (74.4%) |
| Clinical efficacy endpoint at 3 years | 89 (50%) |
| Time related valve safety at 3 years | 85 (81.7%) |
| Hospital stay (days) | 5 ± 4.1 |
| Anesthesia type | |
| Conscious sedation | 282 (78.3%) |
| General anesthesia | 78 (21.7) |
| Implanted valve type | |
| Medtronic Corevalve | 256 (71%) |
| Edwards-Sapien | 94 (26%) |
| Boston Scientific Lotus Valve | 5 (1.4%) |
| Medtronic Corevalve Evolut R | 2 (0.5%) |
| Edwards-Sapien 3 | 3 (0.8%) |
| Access type | |
| Transfemoral | 308 (86.4%) |
| Transapical | 31 (8.6%) |
| Transaxillary | 19 (5.3%) |
| Retroperitoneal | 1 (0.3%) |
| Transaortic | 1 (0.3%) |
There were no significant differences in all-cause mortality nor in cardiovascular mortality rates between patients in whom the transfemoral route was used in comparison with those in whom a nonfemoral access was used (20.1% vs 37.2%, p = 0.2, and 30.6% vs 42.1%, p = 0.1, respectively).
After TAVI, a prominent decrease in the peak pressure gradient and MPG was noted, and at 30 days after valve deployment (from 78.3 ± 24.2 to 15.4 ± 7.6 mm Hg [p >0.01] and from 49.2 ± 16.7 to 8.4 ± 2.7 mm Hg [p >0.01], respectively), this difference was stable at 5-year follow-up ( Figure 2 ).
Moderate and severe PVL was present in 16.4%, 13.4%, and 3.8% of patients at 1-month, 2-year, and 5-year follow-up, respectively. On multivariate analysis, moderate or more paravalvular AR was found to be a strong independent predictor of all-cause mortality (hazard ratio [HR] 4.26, 95% confidence interval [CI] 2.54 to 7.15, p ≤0.001; Table 3 ). This finding explains the stepwise decrease in the rates of significant AR over the follow-up period ( Figure 3 ). Mild PVL was not found to be a predictor of all-cause mortality in univariate analysis (HR 1.03, 95% CI 0.64 to 1.63, p = 0.931). After TAVI, the patients reported sustained symptomatic improvement ( Figure 4 ).
| Variable | Hazard Ratio | 95% Confidence Interval | P value |
|---|---|---|---|
| Hospitalization in the 6 months pre-TAVI | 1.92 | 1.17 – 3.15 | <0.01 |
| Frailty | 1.89 | 1.11 – 3.20 | 0.02 |
| Diabetes Mellitus | 1.54 | 0.93 – 2.56 | 0.10 |
| Glomerular Filtration Rate | 1.00 | 0.99 – 1.01 | 0.71 |
| Baseline Hemoglobin | 0.91 | 0.78 – 1.07 | 0.26 |
| Chronic obstructive pulmonary disease | 1.16 | 0.68 – 2.00 | 0.58 |
| Society of Thoracic Surgeons Score | 1.01 | 0.96 – 1.06 | 0.72 |
| LV EF% | 1.04 | 0.88 – 1.23 | 0.61 |
| Acute Kidney Injury | 1.93 | 1.03 – 3.61 | 0.04 |
| Moderate or Severe Aortic Regurgitation post-TAVI | 4.26 | 2.54 – 7.15 | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


