The present study evaluated the safety and efficacy of percutaneous coronary intervention (PCI) of the unprotected left main coronary artery (ULMCA) for the treatment of cardiac allograft vasculopathy (CAV) in consecutive unselected patients with orthotopic heart transplantation (OHT). PCI in patients with OHT and develop CAV has been associated with greater restenosis rates compared to PCI in patients with native coronary artery disease. A paucity of short- and long-term data is available from patients with OHT who have undergone PCI for ULMCA disease. The present retrospective, multicenter, international registry included 21 patients with OHT and CAV who underwent ULMCA PCI from 1997 to 2009. Angiographic success was achieved in all patients. Drug-eluting stents were used in 14 of the 21 patients. No major adverse cardiac events or repeat OHT occurred within the first 30 days. At a mean follow-up of 4.9 ± 3.2 years, 3 patients (14%) had died, myocardial infarction had occurred in 1 patient (5%), and target lesion revascularization had been required in 4 patients (19%). Follow-up angiography was performed in 16 patients (76%), and restenosis was observed in 4 (19%). No stent thrombosis of the ULMCA was observed. One patient (5%) underwent coronary artery bypass grafting, and 5 patients (24%) underwent repeat OHT. In conclusion, the results of our study have shown ULMCA PCI to be safe and reasonably effective in patients with OHT and represents a viable treatment strategy for CAV in these patients.
Although the standard of care is coronary artery bypass grafting for patients with unprotected left main coronary artery (ULMCA) disease, the 2009 American College of Cardiology/American Heart Association focused guidelines for percutaneous coronary intervention (PCI) have stated that ULMCA PCI is a class IIb recommendation. A paucity of long-term data is available for ULMCA PCI for cardiac allograft vasculopathy (CAV). We therefore examined and report the short- and long-term outcomes of ULMCA PCI for CAV from a multicenter international registry.
Methods
The data from 21 patients with CAV who had undergone ULMCA PCI at the University of California, Los Angeles Medical Center, Los Angeles, California, Stanford Medical Center, Palo Alto, California, University of Padova Medical Center, Padua, Italy, Tampa General Hospital, Tampa, Florida, and Columbia University, New York-Presbyterian Hospital, New York, New York, from 1997 to 2009 were retrospectively collected and reviewed for analysis. The institutional review board at each institution approved the use of patient data for the purposes of the present study.
PCI was performed using standard techniques. All patients who underwent ULMCA PCI from 1997 to 2002 underwent PCI with bare metal stents. In contrast, PCI was performed exclusively with drug-eluting stents at the enrolling centers from 2003 to 2009. The choice of drug-eluting stent (sirolimus-eluting stent, Cypher, Cordis, Johnson & Johnson, Miami, Florida; paclitaxel-eluting stent, Taxus, Boston Scientific, Natick, Massachusetts; or everolimus-eluting stent, Promus, Boston Scientific), anticoagulation, and the use of hemodynamic support devices and intravascular ultrasonography was left to the discretion of the operator. All the patients received both aspirin and ticlopidine or clopidogrel for a minimum of 1 month after bare metal stenting and 6 months after drug-eluting stenting. The transplant cardiologists at the respective institutions determined the immunosuppressive regimens. Annual angiography was performed for 5 years after orthotopic heart transplantation (OHT)—after which, if no significant abnormalities were found, angiography was performed biannually.
The patient data were entered into a computerized database and included the baseline characteristics and clinical or angiographic follow-up results. Surveillance angiography was performed within 6 to 12 months after PCI, or sooner, if clinically indicated. Myocardial infarction was diagnosed according to the presence of new Q waves in ≥2 contiguous electrocardiographic leads and elevated cardiac enzymes. When pathologic Q waves were absent on the electrocardiogram, myocardial infarction was diagnosed when the creatine kinase levels increased to more than twice the upper limit of normal range, with an elevated creatine kinase-MB or troponin I level. Target lesion revascularization (TLR) was defined as repeat revascularization because of restenosis within the stent or in the 5-mm distal or proximal segments. Major adverse cardiac events were defined as the composite of death, myocardial infarction, and TLR. Definite/confirmed stent thrombosis was defined as the presence of an acute coronary syndrome and angiographic confirmation of stent thrombus or occlusion or pathologic confirmation of stent thrombosis. Probable stent thrombosis was defined as any unexplained death within 30 days or as target vessel myocardial infarction without angiographic confirmation of thrombosis or other identified culprit lesion. Possible stent thrombosis was defined as unexplained death after 30 days of the index procedure.
The continuous variables are presented as the mean ± SD and categorical variables as percentages. Survival and major adverse cardiac event-free survival curves were generated using the Kaplan-Meier method. Statistical analysis was performed using the Statistical Package for Social Sciences, version 10.0 (SPSS, Chicago, Illinois).
Results
The baseline clinical characteristics of the 21 patients are listed in Table 1 . The mean age was 54 ± 16 years, and 14 patients (67%) were men. Of the 21 patients, 13 (62%) underwent elective ULMCA PCI. Chronic renal insufficiency (serum creatinine ≥1.5 mg/dl) was present in 10 patients (48%). Cardiogenic shock was present in 3 patients (14%). The mean left ventricular ejection fraction was 51 ± 10%. The mean age of the allograft at PCI was 12 ± 4 years.
| Variable | Value |
|---|---|
| Age at percutaneous coronary intervention (years) | 54 ± 16 |
| Men | 14 (67%) |
| Diabetes mellitus | 7 (33%) |
| Hypertension requiring antihypertensive therapy | 10 (48%) |
| Hypercholesterolemia (total cholesterol >200 mg/dl) | 7 (33%) |
| Chronic renal insufficiency (creatinine ≥1.5 mg/dl) | 10 (48%) |
| Left ventricular ejection fraction | 51 ± 10% |
| Immunosuppressive regimens | |
| Cyclosporine | 10 (48%) |
| Azathioprine | 2 (10%) |
| Mycophenalate | 7 (33%) |
| Prednisone | 17 (81%) |
| Sirolimus | 4 (19%) |
| Tacrolimus | 8 (38%) |
| Indication for percutaneous coronary intervention | |
| Elective | 13 (62%) |
| Urgent/emergent | 8 (38%) |
| Cardiogenic shock | 3 (14%) |
| Age of allograft (years) | 12 ± 4 |
The distal bifurcation of the ULMCA was involved in 10 patients (48%) ( Table 2 ). Heparin was used in all but 1 patient. Glycoprotein IIb/IIIa inhibitors were used in 7 patients (33%). Intravascular ultrasonography was used in 11 patients (52%), and an intra-aortic balloon pump was used in 8 patients (38%). Drug-eluting stents were used in 14 patients (67%). Rotational atherectomy without subsequent stenting was performed in 1 patient (5%). The mean stent diameter was 3.3 ± 0.4 mm, and the mean total stent length was 20 ± 15 mm.
| Variable | |
|---|---|
| Location of left main stenosis | |
| Ostial/midshaft | 11 (52%) |
| Distal bifurcation | 10 (48%) |
| Anticoagulation | |
| Heparin | 20 (95%) |
| Bivalirudin | 1 (5%) |
| Glycoprotein IIb/IIIa inhibitors | 7 (33%) |
| Intra-aortic balloon pump use | 8 (38%) |
| Intravascular ultrasound use | 11 (52%) |
| Percutaneous coronary intervention with drug-eluting stent | 14 (67%) |
| Sirolimus-eluting stent | 10 (48%) |
| Paclitaxel-eluting stent | 3 (14%) |
| Everolimus-eluting stent | 1 (5%) |
| Rotational atherectomy | 1 (5%) |
| Stent diameter (mm) | 3.3 ± 0.4 |
| No. of stents/patient | 1.33 ± 0.49 |
| Total stent length (mm) | 20 ± 15 |
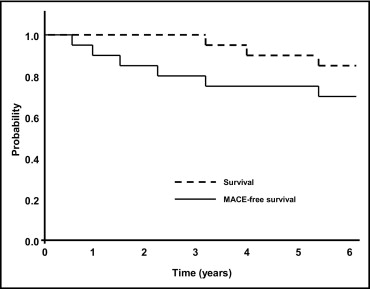
Angiographic success was achieved in 100% of patients. No major adverse cardiac events occurred and repeat OHT was not required within the first 30 days.
At a mean follow-up of 4.9 ± 3.2 years, 3 patients (14%) had died ( Figure 1 and Table 3 ). The only cardiac death occurred in a 60-year-old woman who had died 4.0 years after simultaneous “kissing” stenting of the ULMCA with 2 sirolimus-eluting stents. The autopsy showed stent thrombosis of a nontarget vessel sirolimus-eluting stent. That stent had been implanted in the patient’s right coronary artery 20 days before her death. The other causes of death included multiorgan failure 7 months after coronary artery bypass grafting (3.2 years after ULMCA PCI) and sepsis (5.6 years after ULMCA PCI). One patient (5%) experienced a non–ST-segment elevation myocardial infarction at 5.5 years.
| Patient No. | Age (years) | Gender | Diabetes | Age of Allograft (years) | EF (%) | Stent Type | Lesion Location | MACE | Follow-up (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | Male | No | 14 | 65 | Sirolimus-eluting stent | Distal | TLR | 230 |
| 2 | 23 | Male | No | 5 | 40 | Sirolimus-eluting stent ⁎ | Distal | TLR | 365 |
| 3 | 60 | Female | No | 12 | 40 | Sirolimus-eluting stent ⁎ | Distal |
|
|
| 4 | 43 | Male | No | 15 | 55 | Sirolimus-eluting stent | Midshaft | TLR | 801 |
| 5 | 43 | Female | No | 7 | 65 | Bare metal stent | Ostial | Death | 1,157 |
| 6 | 74 | Male | Yes | 14 | 50 | Sirolimus-eluting stent | Midshaft |
|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


