We compared 5-year clinical outcomes in diabetic and nondiabetic patients treated with Endeavor zotarolimus-eluting stents (ZESs; Endeavor Sprint, Medtronic, Santa Rosa, California) or Cypher sirolimus-eluting stents (SESs; Cordis, Johnson & Johnson, Warren, New Jersey) coronary implantation. We randomized 2,332 patients to either ZESs (n = 1,162, n = 169 diabetic patients) or SESs (n = 1,170, n = 168 diabetic patients) stratified according to presence or absence of diabetes mellitus. End points included major adverse cardiac event (MACE), a composite of cardiac death, myocardial infarction, target vessel revascularization (TVR), and definite stent thrombosis. Among diabetic patients, MACE occurred more frequently in patients treated with ZESs than SESs (48 [28.4%] vs 31 [18.5%]; odds ratio [OR] 1.75, 95% confidence interval [CI] 1.05 to 2.93, p = 0.032) because of a higher rate of TVR (32 [18.9%] vs 14 [8.3%]; OR 2.57, 95% CI 1.32 to 5.02, p = 0.006). Among nondiabetic patients, ZES and SES had similar MACE rates at 5-year follow-up but SES was associated with a significantly higher risk of definite stent thrombosis (10 [1.0%] vs 23 [2.3%]; OR 0.43, 95% CI 0.20 to 0.91, p = 0.028). Moreover, during the last 4 years, ZES had fewer MACE, TVR, and stent thrombosis events among nondiabetic patients. In conclusion, SES remains superior to ZES in patients with diabetes throughout the 5-year follow-up, however, among nondiabetic patients, SES demonstrated a highly dynamic performance with favorable initial results followed by a late catch-up that included an overall higher risk of stent thrombosis.
In patients with diabetes, the drug-eluting stents (DESs) with the lowest late lumen loss (sirolimus- and everolimus-eluting DES) have been associated with the lowest risk of clinical events at short-term follow-up. However, data regarding long-term outcome after implantation of various DES are scarce in diabetic cohorts. In the Danish Organization for Randomized Trials on Clinical Outcome (SORT OUT) III trial, a sirolimus-eluting stent (SES) was compared with a zotarolimus-eluting stent (ZES) in routine clinical care patients. Although the SES is highly effective in inhibiting neointima formation, the ZES is less effective in diabetic patients. In the entire SORT OUT III cohort the 18-month data showed major clinical differences between these DES. Longer-term data performed in selected low-risk populations, however, indicated that the ZES had a lower risk of long-term major adverse cardiac events (MACEs) compared with a paclitaxel-eluting stent. Moreover, the overall outcome in the SORT OUT III trial showed a late catch-up in the SES group and no significant clinical differences at 5-year follow-up. The purpose of the present SORT OUT III substudy was to examine the long-term (5-year) clinical results after implantation of these 2 different DES in patients with and without diabetes.
Methods
SORT OUT III is a multicentre, open-label, randomized, “all-comer” trial that included patients from January 2006 to August 2007 at 5 Danish high-volume percutaneous coronary intervention centers. Inclusion and exclusion criteria have been reported. Patients were considered as diabetic if they received active treatment with insulin, an oral antidiabetic medication, or were treated through diet.
Patients were block randomized and assigned 1:1 to either the ZES (Endeavor Sprint; Medtronic, Santa Rosa, California) or the SES (Cypher Select or Cypher Select+; Cordis, Johnson & Johnson, Warren, New Jersey). Allocation sequence was computer generated and stratified by presence and/or absence of diabetes and gender. The trial complied with the Declaration of Helsinki and was approved by the local ethics committee. All patients provided written informed consent before inclusion.
End point detection and adjudication were obtained as previously outlined. Clinical outcomes in the subgroup analysis were the composite of outcome of MACEs consisting of cardiac death, myocardial infarction, and target vessel revascularization (TVR). Other outcomes included all-cause death, cardiac death, myocardial infarction, TVR, target lesion revascularization (TLR), and angiographically verified (definite) stent thrombosis (ST). Academic Research Consortium definitions were used in adjudication of stent thrombosis.
Distributions of continuous variables in the ZES and SES groups were compared using either the 2-sample t test (or Cochran t test in the case of unequal variance) or the Mann-Whitney U test, depending on whether the data followed a normal distribution. We compared distributions of categorical variables using the chi-square test. We counted end point events occurring during the follow-up period and compared rates for the 2 groups of patients according to presence and/or absence of diabetes. Follow-up began on the date of the index percutaneous coronary intervention procedure. In analyses of each outcome, follow-up continued until the date of an end point event, death, emigration, or until 60 months after implantation, whichever came first. Cumulative incidence curves were constructed based on the cumulative incidence of end point events, taking into account the competing risk of death. Landmark analyses assessing outcomes between >1 and ≤5 years were performed. We estimated differences in groups by calculating odds ratios, except in landmark analysis of definite stent thrombosis in patients without diabetes where risk difference was calculated. SES was used as the reference in all analyses. All analyses were performed according to intention-to-treat principles. A p <0.05 was considered significant. We used SAS software version 9.2 (SAS Institute Inc., Cary, North Carolina) for all analyses.
Results
A total of 2,332 patients were allocated to either ZES (n = 1,162, n = 169 diabetic patients) or SES (n = 1,170, n = 168 diabetic patients) during the inclusion period. Baseline patient and lesion characteristics have been presented previously.
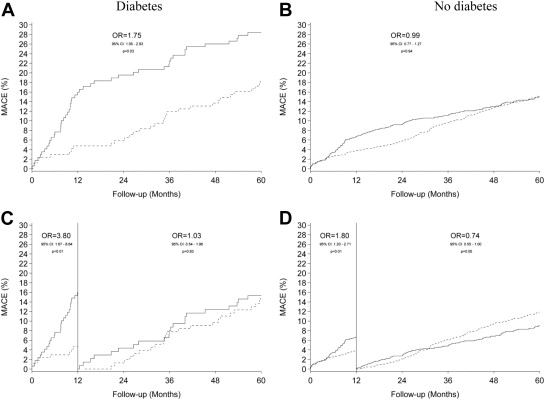
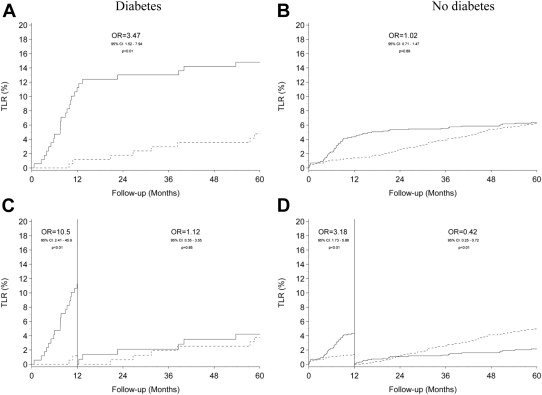
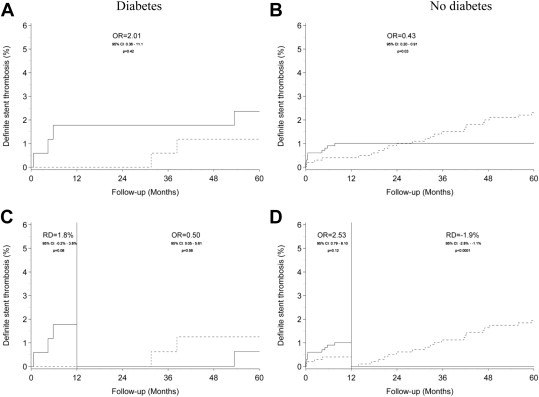
Table 1 details the 5-year clinical outcomes and the landmark results of events occurring >1 year after index intervention. Cumulative event rates of MACE, TLR, and definite stent thrombosis are displayed in Figures 1, 2 , and 3 respectively.
| Patients with diabetes | Patients without diabetes | |||||||
|---|---|---|---|---|---|---|---|---|
| ZES | SES | OR (95% CI) | p Value | ZES | SES | OR (95% CI) | p Value | |
| 0-60 MONTHS | ||||||||
| MACE | 48 (28.4%) | 31 (18.5%) | 1.75 (1.05-2.93) | 0.0322 | 149 (15.0%) | 151 (15.1%) | 0.99 (0.77-1.27) | 0.94 |
| All-cause death | 27(16.0%) | 30 (17.9%) | 0.87 (0.49-1.55) | 0.65 | 126 (12.7%) | 108 (10.8%) | 1.20 (0.91-1.57) | 0.20 |
| Cardiac death | 11 (6.5%) | 12 (7.1%) | 0.91 (0.39-2.11) | 0.82 | 37 (3.7%) | 42 (4.2%) | 0.88 (0.56-1.38) | 0.58 |
| Myocardial infarction | 15 (8.9%) | 12 (7.1%) | 1.27 (0.57-2.79) | 0.56 | 49 (5.0%) | 54 (5.4%) | 0.91 (0.61-1.35) | 0.64 |
| Definite stent thrombosis | 4 (2.4%) | 2 (1.2%) | 2.01 (0.36-11.1) | 0.42 | 10 (1.0%) | 23 (2.3%) | 0.43 (0.20-0.91) | 0.0278 |
| Target lesion revascularization | 25 (14.8%) | 8 (4.8%) | 3.47 (1.52-7.94) | 0.0032 | 63 (6.4%) | 62 (6.2%) | 1.02 (0.71-1.47) | 0.89 |
| Target vessel revascularization | 32 (18.9%) | 14 (8.3%) | 2.57 (1.32-5.02) | 0.0057 | 89 (9.0%) | 97 (9.7%) | 0.92 (0.68 -1.24) | 0.57 |
| 12-60 MONTHS | ||||||||
| MACE | 21 (15.3%) | 23 (14.9%) | 1.03 (0.54-1.96) | 0.92 | 83 (9.1%) | 113 (11.9%) | 0.74 (0.55-1.00) | 0.0483 |
| All-cause death | 18 (11.3%) | 22 (13.8%) | 0.80 (0.41-1.55) | 0.50 | 104 (10.7%) | 92 (9.4%) | 1.16 (0.86-1.56) | 0.32 |
| Cardiac death | 7 (4.4%) | 10 (6.3%) | 0.69 (0.25-1.85) | 0.46 | 27 (2.8%) | 33 (3.4%) | 0.82 (0.49-1.38) | 0.46 |
| Myocardial infarction | 8 (5.2%) | 11 (6.9%) | 0.74 (0.29-1.89) | 0.52 | 35 (3.7%) | 49 (5.0%) | 0.72 (0.46-1.12) | 0.14 |
| Definite stent thrombosis | 1 (0.6%) | 2 (1.3%) | 0.50 (0.05-5.61) | 0.58 | 0 (0.0%) | 19 (1.9%) | <0.0001 ∗ | |
| Target lesion revascularization | 6 (4.2%) | 6 (3.8%) | 1.12 (0.35-3.55) | 0.85 | 20 (2.2%) | 48 (5.0%) | 0.42 (0.25-0.72) | 0.0014 |
| Target vessel revascularization | 10 (7.2%) | 9 (5.8%) | 1.26 (0.50-3.19) | 0.63 | 33 (3.6%) | 69 (7.2%) | 0.48 ( (0.31-0.73) | 0.0007 |

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


