Recently, moderate and severe postprocedure aortic regurgitations (ARs) have been identified as independent risk factors for short- and midterm mortality after transcatheter aortic valve implantation (TAVI). However, very few data exist on the long-term outcome of postprocedure AR. From 2008 to 2011, 198 consecutive patients with severe aortic stenosis successfully underwent TAVI with the CoreValve prosthesis (Medtronic CV, Minneapolis, Minnesota). After the procedure, patients were subdivided into groups depending on the presence of moderate/severe AR. The primary study end point was death from any cause after TAVI. The secondary end point was defined as cardiovascular death. In study patients (80 ± 6 years old, logistic European System for Cardiac Operative Risk Evaluation 22 ± 16%, left ventricular ejection fraction 53 ± 13%), moderate/severe AR occurred in 28 patients (14%). Despite similar baseline characteristics, patients with moderate/severe AR had higher 30-day and 1-year mortality rates than patients with none/mild AR (21% vs 6%, p = 0.019; 57% vs 16%, p <0.001, respectively). During a mean follow-up of 535 ± 333 days, the primary end point was reached in 54 and the secondary end point in 33 patients. Moderate/severe AR was the strongest independent risk factor of all-cause-mortality (hazard ratio 4.89, 95% confidence interval 2.78 to 8.56, p <0.001) and the strongest independent risk factor of cardiovascular mortality (hazard ratio 7.90, 95% confidence interval 3.95 to 15.81, p <0.001). In conclusion, moderate and severe postprocedure ARs are not uncommon complications after TAVI. Although long-term outcome of patients with none/mild AR is favorable, outcome of patients with moderate/severe AR is dismal.
Recently, moderate and severe prosthetic aortic regurgitation (AR) has been identified as an independent risk factor for short- and midterm mortality after transcatheter aortic valve implantation (TAVI). Incongruence between device and aortic annulus, extensive calcification of the aortic valve, and malposition of the prosthesis are considered the main causes of AR after TAVI. However, very few data exist on the long-term course and outcome of postprocedure AR. The purpose of the present study was to investigate the incidence of AR after TAVI with the CoreValve prosthesis (Medtronic CV, Minneapolis, Minnesota) and its impact on functional status and long-term outcome.
Methods
Patients with symptomatic, severe aortic stenosis (aortic valve area ≤1.0 cm 2 ) and high risk for surgical aortic valve replacement were screened for transfemoral or transaxillary TAVI. Risk of surgical aortic valve replacement was estimated with the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE). Indications, contraindications, and anatomic requirements for TAVI were described previously.
After the procedure patients were subdivided into groups depending on the presence of moderate/severe AR at echocardiographic examination. All patients gave informed consent before participation. The protocol was approved by the local ethics committee.
The primary study end point of this prospective single-center investigation was death from any cause after TAVI. The secondary end point was defined as cardiovascular death. Cardiovascular death included death from myocardial infarction, stroke, sudden cardiac death, heart failure, fatal prosthesis failure with conversion to heart surgery, and death of unknown cause. Study end points were defined according to recommendations of the Valve Academic Research Consortium. In addition, functional status and hemodynamics of the prosthesis were investigated. All patients were followed for ≥1 year. Follow-up information was obtained during routine ambulatory visits and by telephone contact with the deceased patients’ physicians.
The aortic annulus was measured using preprocedural transthoracic and/or transesophageal echocardiography. In addition, in 152 patients (77%) measurement of aortic annulus was performed by multislice computed tomography. Valve sizes of 26- and 29-mm expanded diameters were employed. Prosthesis size was determined by consensus at the interventional team after reviewing all imaging techniques. The aortic valve prosthesis (18Fr generation, Medtronic CoreValve Percutaneous System, Medtronic CV) was inserted retrograde without hemodynamic support. Procedural characteristics have been described in detail. To assess the congruence between the aortic annulus and the prosthesis, we used a recently described “cover index” expressed as a ratio of 100 × ([prosthesis diameter minus annulus diameter {echocardiography}]/prosthesis diameter). Prosthesis/annulus incongruence was considered if the cover index was ≤8.
According to current recommendations, the ventricular edge of the prosthesis should be placed 5 to 10 mm below the aortic valve annular plane. A too-low implantation was defined as the distal edge of the valve positioned >10 mm below the annulus level. A too-high implantation was defined as the distal edge of the valve positioned above the aortic annulus. Inadequate expansion of prosthesis was defined as deformation of the prosthesis owing to annulus calcification despite a correct position ( Figure 1 ). Intraprocedural AR was assessed by supra-aortic angiography.
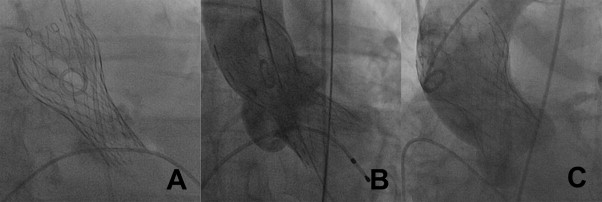
Immediately after implantation, invasive measurements were performed. Retrospectively, we calculated the recently described “aortic regurgitation index” expressed as a ratio of ([diastolic blood pressure minus left ventricular end-diastolic pressure]/systolic blood pressure).
Echocardiographic examinations of patients were performed within 7 days before TAVI, within 5 days after TAVI, at 30 days, at 12 months, and annually thereafter. Transthoracic echocardiography was performed according to guidelines of the American Society of Echocardiography using a digital ultrasound scanner (Vivid 7, General Electrics, Horton, Norway). After TAVI, prosthetic valve performance was evaluated according to recommendations of the Valve Academic Research Consortium. Prosthetic aortic valve regurgitation was graded by echocardiography as mild, moderate, and severe. Changes in left ventricular geometry and function were documented at follow-up examinations. Interobserver and intraobserver correlations for echocardiographic measurements reached 0.93 and 0.97, respectively.
Numerical values are expressed as mean ± SD. Continuous variables were compared between groups using unpaired t test (for normally distributed variables) or Mann–Whitney U test (for non-normally distributed variables). Continuous variables were compared between patients before and after TAVI using paired Student’s t test (for normally distributed variables) or Wilcoxon test (for non-normally distributed variables). All variables lists in Table 1 and postprocedure AR were evaluated for all-cause mortality and cardiovascular mortality in univariate regression analysis. All variables with a significant association with end points (p <0.05) were entered in multivariate regression analysis to identify independent predictors of all-cause mortality and cardiovascular mortality. Results are present as hazard ratio (HR). For outcome analysis, Kaplan–Meier survival curves were generated and differences between all-cause mortality and cardiovascular mortality were assessed using log-rank test. All reported probability values are 2-tailed and p <0.05 was considered statistically significant. Analyses were performed with the SPSS 19.0 (SPSS, Inc., Chicago, Illinois).
| Variable | All | None/mild AR | Moderate/Severe AR | p Value |
|---|---|---|---|---|
| (n = 170) | (n = 28) | |||
| Age (years) | 80 ± 6 | 79 ± 6 | 80 ± 6 | 0.378 |
| Women | 105 (53%) | 93 (54%) | 14 (50%) | 0.839 |
| Logistic EuroSCORE (%) | 22 ± 16 | 21 ± 16 | 24 ± 18 | 0.317 |
| New York Heart Association functional class III or IV | 187 (94%) | 160 (94%) | 27 (96%) | 1.000 |
| Body mass index (kg/m 2 ) | 27 ± 5 | 27 ± 5 | 27 ± 5 | 0.895 |
| Coronary artery disease | 107 (52%) | 91 (53%) | 13 (46%) | 0.543 |
| Previous myocardial infarction | 43 (21%) | 36 (21%) | 7 (25%) | 0.627 |
| Previous coronary artery bypass grafting | 31 (16%) | 27 (16%) | 4 (14%) | 1.000 |
| Atrial fibrillation | 66 (33%) | 57 (34%) | 9 (32%) | 1.000 |
| Mitral regurgitation (moderate/severe) | 87 (44%) | 73 (43%) | 14 (50%) | 0.533 |
| Chronic obstructive pulmonary disease | 59 (30%) | 48 (28%) | 11 (39%) | 0.267 |
| Creatinine (mg/dl) | 1.2 ± 0.7 | 1.2 ± 0.7 | 1.3 ± 0.9 | 0.231 |
| Aortic valve area (cm 2 ) | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.654 |
| Aortic mean gradient (mm Hg) | 47 ± 13 | 47 ± 13 | 44 ± 13 | 0.198 |
| Left ventricular end-diastolic volume (ml) | 116 ± 36 | 115 ± 36 | 125 ± 38 | 0.175 |
| Left ventricular end-systolic volume (ml) | 51 ± 28 | 50 ± 29 | 57 ± 27 | 0.194 |
| Left ventricular ejection fraction (%) | 53 ± 13 | 54 ± 13 | 50 ± 11 | 0.108 |
Results
In total 202 consecutive patients with native aortic stenosis (n = 197) or degenerated aortic bioprosthesis (n = 5) who underwent transfemoral (n = 197) or transaxillary (n = 5) TAVI with the CoreValve prosthesis were enrolled.
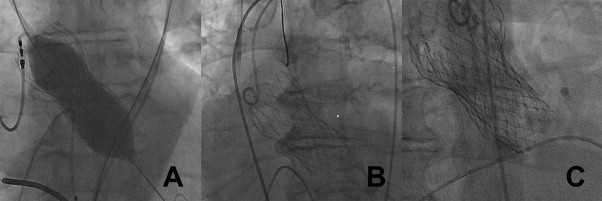
During the procedure 3 patients died (rupture of eccentric calcified aortic base, n = 1; fatal vascular access-related complications, n = 2). These patients died before the prosthesis was implanted and evaluation of AR was not possible. Hence, these patients were excluded from main analysis. Owing to severe AR after implantation, 2 patients were converted to surgery. Of them, 1 patient survived and was excluded from main analysis. Procedural complications are listed in Table 2 and baseline characteristics of patients are provided in Table 1 . Intraprocedural reinterventions ( Table 2 , Figure 2 ) improved degree of AR in 12 patients but remained unchanged in 8 patients.
| Patients (n) | |
|---|---|
| Procedural complications | |
| Death | 3 |
| Conversion to surgery | 2 |
| Myocardial infarction | 2 |
| Stroke | 4 |
| Renal failure requiring hemodialysis | 5 |
| Pericardial tamponade | 6 |
| Surgical vascular repair | 23 |
| Intraprocedural reinterventions | |
| Balloon redilatation | 18 |
| Retraction of prosthesis and second implantation owing to valve dislocation | 9 |
| Repositioning by snare catheter | 5 |
| Implantation of second prosthesis owing to valve embolization | 3 |
| Prosthesis-in-prosthesis implantation | 2 |
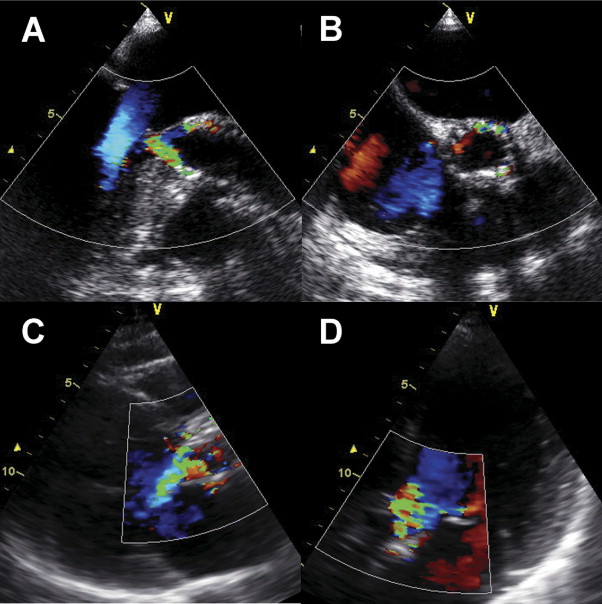
At echocardiographic examination after TAVI, any prosthetic valve regurgitation occurred in 115 of 198 patients (58%; Figure 3 ). Of them, 111 patients had paravalvular regurgitation and 4 patients presented with transvalvular regurgitation. Overall, mild AR occurred in 83 patients (43%), moderate AR in 24 patients (12%), and severe AR in 4 patients (2%; Figure 4 ). There were no significant differences in baseline characteristics of patients with or without moderate/severe AR ( Table 1 ). In 28 patients with moderate/severe AR we retrospectively evaluated reasons of AR: incomplete apposition of prosthesis from asymmetrical or severe calcification of the aortic annulus (n = 15), too-low implantation (n = 7), too-high implantation (n = 2), prosthesis/annulus incongruence (n = 3), and central transvalvular regurgitation after balloon redilatation (n = 1). Anatomic and hemodynamic parameters are presented in Table 3 .
| Variable | None/Mild AR | Moderate/Severe AR | p Value |
|---|---|---|---|
| Annulus aorta (echocardiography) (mm) | 23.5 ± 2 | 24.3 ± 2.1 | 0.067 |
| Annulus aorta (computed tomography) (mm) ⁎ | 24.2 ± 2.2 | 24.6 ± 1.9 | 0.558 |
| Prosthesis size (29 mm) | 115 (71%) | 20 (71%) | 0.656 |
| Cover index (%) † | 15.9 ± 6.2 | 13.7 ± 6 | 0.084 |
| Position of prosthesis (mm) ‡ | 6.7 ± 2.8 | 8.3 ± 3.4 | 0.029 |
| Aortic regurgitation index § | 25.6 ± 10 | 20.2 ± 10 | 0.013 |
⁎ Measurement in 152 patients who underwent multislice computed tomography.
† Ratio of: 100 × ([prosthesis diameter minus annulus diameter {echocardiography}]/prosthesis diameter).
‡ Distance between ventricular edge of the prosthesis and aortic valve annular plane.
§ Aortic regurgitation index: [(diastolic blood pressure minus left ventricular end-diastolic pressure)/systolic blood pressure).
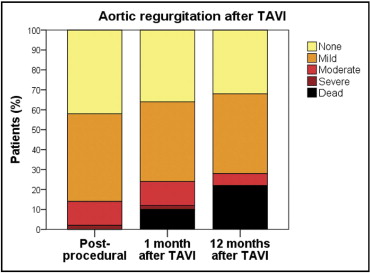
Changes in left ventricular diameters and function 30 days after TAVI are presented in Table 4 . At 1-year follow-up, differences in patient subgroups did not reach significant relevance. Probably this was due to the small group of patients with moderate/severe AR surviving the first year after TAVI. Changes in degree of AR are presented in Figure 5 .
| None/Mild AR | Moderate/Severe AR | p Value | |
|---|---|---|---|
| Δ Aortic valve area (cm 2 ) | 1.1 ± 0.3 | 1.1 ± 0.4 | 0.821 |
| Δ Aortic mean gradient (mm Hg) | −39 ± 12 | −35 ± 14 | 0.301 |
| Δ Left ventricular end-diastolic volume (ml) | −10 ± 30 | 7 ± 25 | 0.016 |
| Δ Left ventricular end-systolic volume (ml) | −5 ± 20 | −1 ± 19 | 0.388 |
| Δ Left ventricular ejection fraction (%) | 2 ± 10 | 2 ± 15 | 0.884 |
| Mitral regurgitation—improvement | 45 (26%) | 2 (7%) | 0.069 |
| Mitral regurgitation—worsening | 23 (14%) | 7 (25%) | 0.061 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


