Super-response to cardiac resynchronization therapy (CRT) is associated with significant left ventricular (LV) reverse remodeling and improved clinical outcome. The study aimed to: (1) evaluate whether LV reverse remodeling remains sustained during long-term follow-up in super-responders and (2) analyze the association between the course of LV reverse remodeling and ventricular arrhythmias. Of all, primary prevention super-responders to CRT were selected. Super-response was defined as LV end-systolic volume reduction of ≥30% 6 months after device implantation. Cox regression analysis was performed to investigate the association of LV ejection fraction (LVEF) as time-dependent variable with implantable-cardioverter defibrillator (ICD) therapy and mortality. A total of 171 super-responders to CRT-defibrillator were included (mean age 67 ± 9 years; 66% men; 37% ischemic heart disease). Here of 129 patients received at least 1 echocardiographic evaluation after a median follow-up of 62 months (25th to 75th percentile, 38 to 87). LV end-diastolic volume, LV end-systolic volume, and LVEF after 6-month follow-up were comparable with those after 62-month follow-up (p = 0.90, p = 0.37, and p = 0.55, respectively). Changes in LVEF during follow-up in super-responders were independently associated with appropriate ICD therapy (hazard ratio 0.94, 95% CI 0.90 to 0.98; p = 0.005) and all-cause mortality (hazard ratio 0.95, 95% CI 0.91 to 1.00; p = 0.04). A 5% increase in LVEF was associated with a 1.37 times lower risk of appropriate ICD therapy and a 1.30 times lower risk of mortality. In conclusion, LV reverse remodeling in super-responders to CRT remains sustained during long-term follow-up. Changes in LVEF during follow-up were associated with mortality and ICD therapy.
Cardiac resynchronization therapy (CRT) reduces mortality and morbidity in patients with advanced heart failure, reduced left ventricular (LV) function, and prolonged QRS duration. In general, CRT implantation induces LV reverse remodeling. However, patients experience this response to CRT in varying degrees. Women, patients with nonischemic heart disease, a prolonged QRS duration, and/or left bundle branch block (LBBB) are most likely to experience more pronounced LV reverse remodeling.
Furthermore, patients who experience most pronounced LV reverse remodeling and (near) normalization of systolic heart function, so called super-responders, have most advantageous clinical outcome and survival. Furthermore, the risk of ventricular arrhythmias is reduced after CRT implantation; it is of clinical importance to determine whether super-responders still require implantable-cardioverter defibrillator (ICD) treatment. We previously reported that super-responders required ICD therapies especially after long-term follow-up. On the one hand, it can be expected that with (near) normalization of the systolic heart function the increased risk of ventricular arrhythmias disappears, in contrast, CRT implantation often leaves the pathological substrate untreated.
Subsequently, it is unexplored whether possible ventricular arrhythmias during long-term follow-up are associated with patients’ deteriorating. Therefore, the aim of this study was twofold; (1) to evaluate LV reverse remodeling in super-responders during long-term follow-up and (2) to analyze the association between the course of LV volume or function and ventricular arrhythmias or survival.
Methods
All patients who received a CRT-defibrillator (CRT-D) device at the LUMC (the Netherlands) from January 2000 to May 2012 were recorded in the departmental Cardiology Information System (EPD-vision, LUMC). Eligibility for the device was based on the current international guidelines. For the present study, only echocardiographic super-responders to CRT were included. Super-response was defined as decreased LV end-systolic volume (LVESV) ≥30% determined 6 months after implantation. In addition, patients were excluded from the study when one of the following criteria was met: secondary prevention ICD implantation; Cardiac Resynchronization Therapy-Pacemaker treatment before CRT-D implantation; congenital or monogenetic heart disease; decompensated heart failure at the time of device implantation; myocardial infarction <3 months before device implantation; or LV reconstructive surgery.
Follow-up visits were planned periodically every 3 to 6 months or more frequently when clinically indicated. If follow-up visits were not performed for more than 6 months, follow-up was considered incomplete. These patients were included in the current analysis up to their final outpatient follow-up visit. All consecutive follow-up visits were prospectively registered up to January 2015. Follow-up visits included clinical assessment and device interrogation performed under supervision of electrophysiologists or device cardiologists. Echocardiographic evaluation was only performed when clinically indicated at the discretion of the treating physician.
During device interrogation, device printouts were analyzed and ICD therapies were registered. ICD therapies were classified using intracardial electrograms. ICD therapy (antitachycardia pacing and shock) was only considered appropriate when occurring in response to sustained ventricular tachycardia or ventricular fibrillation, otherwise ICD therapy was considered inappropriate (triggered by sinus or supraventricular tachycardia, nonsustained ventricular arrhythmias, T-wave oversensing or lead dysfunction). Appropriate ICD therapy or appropriate ICD shock were considered the primary end point and all-cause mortality, the secondary end point.
All CRT-D devices were implanted transvenously. The devices used were manufactured by Biotronik (Berlin, Germany), Boston Scientific (Natick, Massachusetts, formerly CPI, Guidant [St. Paul, Minnesota]), Medtronic (Minneapolis, Minnesota), or St Jude Medical/Ventritex (St. Paul, Minnesota).
In general, the defibrillators were programmed with 3 different zones: ventricular arrhythmias from 150 to 155 beats/min to 185 to 190 beats/min were detected in a monitoring zone in which no therapy was programmed (30 to 32 intervals were needed for detection or 8/10 with a 2.5-second [s] initial delay depending on the manufacturer); ventricular arrhythmias faster than 185 to 190 beats/min were programmed with 2 bursts to terminate the arrhythmias, followed by shock if the arrhythmias continue (22 to 30 intervals were needed for detection or 8/10 with a 2.5-second initial delay depending on the manufacturer). In the final zone programmed to detect arrhythmias exceeding 205 to 210 beats/min, shock was the primary therapy (12 to 30 intervals were needed for detection or 8/10 with a 1.0-second initial delay depending on the manufacturer). Zone limit were adjusted when clinically indicated. Furthermore, supraventricular tachycardia discriminators were enabled and atrial arrhythmia detection was set to >170 beats/min.
Before and 6 months after CRT-D implantation, all patients underwent comprehensive echocardiographic evaluation. During follow-up, echocardiographic evaluations were repeated when clinically indicated at the discretion of the treating physician and following a systematic data acquisition protocol. The echocardiographic evaluation performed 6 months after implantation is defined short-term echocardiographic outcome, and from that time to the last additional echocardiographic evaluation is defined as long-term echocardiographic outcome.
Two-dimensional and color Doppler echocardiographic images were obtained using commercially available systems (Vivid 7 and E9, GE-Vingmed Ultrasound, Horten, Norway) equipped with 3.5-MHZ and M5S transducers. Measurement of the LV end-diastolic volume (LVEDV), LVESV, and LV ejection fraction (LVEF) was based on the Simpson’s method using the apical 2- and 4-chamber views (EchoPac 113, GE Medical Systems, Horten, Norway).
The current analysis includes echocardiographic super-responders of CRT, as determined by the extent of LV reverse remodeling. This subgroup was predefined as CRT-D recipients with a decreased LVESV ≥30% 6 months after implantation. After long-term follow-up, the response status was reevaluated by comparing baseline and long-term echocardiographic evaluation, subgroups were negative responders (LVESV increase), nonresponders (LVESV decrease 0% to 15%), responders (LVESV decrease 15% to 30%), and super-responders.
Statistical analyses were performed using the statistical software program IBM SPSS Statistics 20 (Chicago, Illinois). Dichotomous variables are expressed as numbers and percentages. Continuous variables are presented as mean with SD or median with 25th to 75th percentile when indicated. The first echocardiographic evaluation performed in every consecutive 6-month timeframe was analyzed. Short-term and long-term echocardiographic measurements were compared using a linear mixed model with unstructured covariance structure for residuals. A value of p ≤0.05 is considered significant.
Cumulative event rates with a corresponding 95% 95% CI were calculated using Kaplan–Meier analysis event rates were compared using log-rank statistics. Furthermore, a Cox regression model was constructed including LVEDV, LVESV, or LVEF as time-dependent variable. The time-dependent variable allowed the echocardiographic parameters to change every 6 months. In case of missing values, the last known value was included in the following time frame. The multivariate Cox regression analysis adjusted the predefined clinical parameters which included age, gender, and etiology of heart failure, LBBB, New York Heart Association functional class, a history of atrial fibrillation or flutter, renal clearance, and diabetes, for the primary end point (ICD therapy and ICD shock). The secondary end point (mortality) was also adjusted for ICD shocks.
Results
As illustrated in Table 1 , this study includes 171 super-responders to CRT. Patients had a mean age of 67 ± 9 years, the majority was men (66%) and had nonischemic heart disease (63%). Most patients suffered from symptoms of advanced heart failure (New York Heart Association ≥III: 73%), with reduced LVEF (24 ± 6%) and prolonged QRS duration (170 ± 20 ms) most frequently combined with LBBB (80%). The mean percentage of biventricular pacing was 97 ± 8%, measured 6 months after implantation.
| All Included (N=171) | |
|---|---|
| Clinical parameters | |
| Age (years) | 67±9 |
| Men | 112 (66%) |
| BMI (kg/m 2 ) | 26±4 |
| Coronary heart disease | 63 (37%) |
| NYHA functional class | |
| II | 47 (27%) |
| III | 114 (67%) |
| IV | 10 (6%) |
| QRS duration (ms) | 170±20 |
| Left bundle branch block | 137 (80%) |
| Atrial fibrillation/flutter (History) | 51 (30%) |
| Creatinine clearance (ml/min) | 73±30 |
| Hypertension | 81 (47%) |
| Diabetes Mellitus | 33 (19%) |
| Smoker | 65 (38%) |
| Echocardiographic parameters | |
| Left ventricular end diastolic volume (ml) | 220±72 |
| Left ventricular end systolic volume (ml) | 168± 61 |
| Left ventricular ejection fraction (%) | 24±6 |
| Medication | |
| ACE inhibitors /AT II antagonists | 152 (89%) |
| Aldactone | 80 (47%) |
| Amiodarone | 24 (14%) |
| Beta-blockers | 128 (75%) |
| Sotalol | 15 (9%) |
| Calcium antagonists | 12 (7%) |
| Diuretics | 137 (80%) |
| Statins | 89 (52%) |
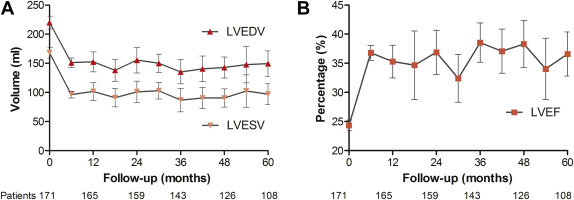
All 171 patients received echocardiographic evaluation before and 6 months after CRT-D implantation. Figure 1 illustrates the results of long-term echocardiographic follow-up. Before implantation, patients had a mean LVEDV of 220 ± 72 ml and a mean LVESV of 168 ± 61 ml, which reduced to 151 ± 53 ml and 96 ± 38 ml, respectively 6 months after implantation. The mean LVEF was increased from 24 ± 6% to 37 ± 9% at 6-month follow-up ( Table 2 ).
| Baseline | 6 months | 62 months (38–87 months) | Estimated mean difference (95% CI) | p-value | |
|---|---|---|---|---|---|
| All (N=171) | |||||
| Left ventricular end diastolic volume (ml) | 220±72 | 151±53 | 150±63 | 0.58 (-8.80–9.96) | 0.90 |
| Left ventricular end systolic volume (ml) | 168±61 | 96±38 | 99±55 | 3.86 (-4.66–12.39) | 0.37 |
| Left ventricular ejection fraction (%) | 24±6 | 37±9 | 36±11 | -0.63 (-2.88–1.61) | 0.55 |
Long-term echocardiographic evaluation was not available in 42 patients. In 22 patients, the follow-up was performed elsewhere, 11 patients died within 30 months after device implantation, and in 9 patients, the treating physician did not request a new echocardiography. The remaining 129 patients received at least 1 additional echocardiographic examination. The last evaluation in these patients was performed after a median follow-up of 62 months (25th to 75th percentile, 38 to 87). As illustrated by Table 2 , long-term echocardiographic outcomes were comparable with those at 6-month follow-up.
Subgroup analyses of echocardiographic outcome at 6 and 62 months are illustrated in Figure 2 . Results were stratified for gender, etiology of heart failure, presence of LBBB, and diabetes. In most subgroups, LV volumes and function were sustained from 6 to 62 months. In patients with ischemic heart disease, however, LVESV was significantly increased from 98 ± 40 to 117 ± 62 ml. Subsequently, a nonsignificant decrease in LVEF was observed. A similar deterioration was observed in patients with non-LBBB morphology. LVESV increased significantly from 94 ± 35 to 119 ± 65 ml, and LVEF decreased from 38 ± 9% to 32 ± 10%. In patients with diabetes, LVESV increased from 96 ± 31 to 113 ± 54 ml, and LVEF decreased from 36 ± 9% to 30 ± 9%.
Compared with the 6-month measurements, LVESV at long-term follow-up was unchanged or further decreased in 52 patients (40%). In the remaining 77 patients (60%), the mean LVESV increase was 34 ± 36 ml. In these deteriorated patients, no acute ischemic events had occurred during follow-up, 5 (6%) underwent elective percutaneous coronary intervention without troponin increase afterward and 1 (1%) an elective coronary artery bypass grafting. In the patient group with sustained LVESV, 1 patient (2%) underwent elective percutaneous coronary intervention. In addition, no association was observed between the percentage of biventricular pacing and patients deteriorating after long-term follow-up (p = 0.27).
As illustrated in Figure 3 , the super-response to CRT was sustained in 83 patients (64%) after long-term follow-up, 19 (15%) were still responders. Yet, 27 patients (21%) deteriorated to nonresponse (9%) or negative response (12%) status.
The median follow-up was 81 months (25th to 75th percentile, 60 to 117) in the entire study population. During follow-up, 40 super-responders (23%) received appropriate device therapy. Appropriate shock alone, was experienced by 24 patients with (13%). The 5-year cumulative incidence of ICD therapy and ICD shock were respectively 22% (95% CI 15% to 28%) and 10% (95% CI 5% to 15%).
To analyze the association of LVEDV, LVESV, or LVEF during follow-up and the risk of appropriate ICD therapy or appropriate shock, these parameters were implemented as a time-dependent variable in a Cox regression model ( Table 3 ). LVEDV change was not associated with ICD therapy or ICD shock. Furthermore, a trend was observed that LVESV during follow-up was associated with risk of ICD therapy, also after adjustment for the predefined covariates (hazard ratio [HR] 1.06, 95% CI 1.00 to 1.15; p = 0.10) but not with ICD shock alone (HR 1.04, 95% CI 0.94 to 1.16; p = 0.45). Increased LVEF during follow-up, however, was associated with reduced risk of ICD therapy (HR 0.94, 95% CI 0.90 to 0.98; p = 0.002) and reduced risk of ICD shock (HR 0.94, 95% CI 0.89 to 0.99; p = 0.03). After adjustment for the predefined covariates, an independent association between LVEF and ICD therapy remained (HR 0.94, 95% CI 0.90 to 0.98; p = 0.005 and HR 0.94, 95% CI 0.88 to 1.00; p = 0.07, respectively).



