Although the benefits of implantable cardioverter-defibrillator (ICD) therapy in patients with Brugada syndrome (BrS) at risk of sudden cardiac arrest are well established, these relatively young patients can encounter complications in the long term. The objective of the present study was to determine the incidence of device complications in patients with BrS during long-term follow-up. The prevalence of device-related complications and the clinical outcome were determined in 41 consecutive patients with BrS (age 48 ± 12 years; 38 men) who were treated with ICD implantation. During a median follow-up of 76 months (interquartile range 51 to 98), 15 patients (37%) experienced 21 adverse events, including 11 device-related complications in 8 (20%) and ≥1 inappropriate shock in 10 (24%). Five patients (12%) received appropriate shocks for ventricular fibrillation. Excluding inappropriate shocks, 95.1%, 89.6%, 86.6%, and 73.3% of patients were free of device-related complications at 1, 3, 5, and 7 years, respectively. Also, 92.6%, 89.8%, 89.9%, and 86.1% were free of lethal arrhythmias at 1, 3, 5, and 7 years, respectively. Adverse effects related to ICD implantation are not uncommon, and their prevalence increases with the follow-up duration required for patients with BrS. In conclusion, because complications can be encountered even very late after device implantation, asymptomatic young patients should be carefully selected and educated about the long-term outcomes of ICD therapy.
Although the implantable cardioverter-defibrillator (ICD) treatment rate will continue to increase in the future, device implantation is not free of complications. Brugada syndrome (BrS) is associated with an increased risk of sudden cardiac death from ventricular tachyarrhythmias. An ICD is often recommended for those at high risk. However, some studies have shown a high incidence of inappropriate shocks in this subset of ICD recipients. Because the ICD-eligible BrS population is relatively young and is expected to live longer after device implantation, the risks and benefits of ICD therapy should be discussed before ICD implantation. The main objective of the present study was to describe device-related complications in relation to the clinical outcomes during long-term follow-up in patients with BrS, who have been treated with ICD.
Methods
The study population consisted of 41 consecutive Japanese patients with Brugada type 1 findings on the electrocardiogram in the absence or presence of a sodium channel–blocking agent who underwent ICD implantation from 2002 to 2011. The diagnosis of BrS was according to the report of the second consensus conference. We excluded conditions mimicking BrS by undertaking the following investigations: laboratory tests to exclude acute cardiac ischemia and metabolic and electrolyte disturbances; echocardiography and, if indicated, stress testing and coronary angiography to rule out structural heart disease. ICD implantation was directed by an experienced electrophysiologist. All patients were examined at 3-month intervals at our institution. A total of 41 consecutive patients who had undergone ICD implantation during the same period for structural heart disease were included as a control group.
Electrophysiologic testing was performed in 35 patients (85%) in a nonsedated, postabsorptive state, with and without a history of sudden cardiac death, who agreed to participate in the present study. A quadripolar catheter was advanced by way of the femoral vein. Programmed stimulation was performed with ≤3 extrastimuli after basic drive cycle lengths of 600 and 400 ms sequentially from the right ventricular apex and outflow tract. The end point of programmed stimulation was sustained ventricular tachycardia or ventricular fibrillation (VF) requiring cardioversion/defibrillation or the delivery of the extrastimuli at the coupling interval decrementing ≤180 ms or local refractoriness, whichever occurred first. We recommended ICD therapy for patients with sustained VF inducible by any level of programmed stimulation.
In 26 patients (63%), 1.0 mg/kg pilsicainide was administered intravenously within 10 minutes. The 12-lead electrocardiogram was monitored for ST-segment changes of type I BrS. The test was considered positive if a type I Brugada pattern was observed.
All patients underwent ICD interrogation every 3 months. No patient was lost to follow-up. Appropriate ICD shocks were defined as shocks delivered for ventricular tachycardia or VF. Inappropriate ICD shocks were defined as shocks delivered in the absence of documented ventricular arrhythmias. Inappropriate shocks due to lead fracture were not counted as inappropriate shocks in the present study.
Continuous variables are expressed as the mean ± SD or the median and interquartile range, depending on the normality of distribution. Continuous and categorical variables were compared using Student’s t test and the chi-square test, respectively. The event rates were determined using the Kaplan-Meier method. The difference in complication-free survival was evaluated using the log-rank test. A p value <0.05 indicated statistical significance.
Results
The clinical characteristics and indications for ICD implantation are listed in Tables 1 and 2 . The patient age at implant was significantly younger in the BrS group than in the control group.
| Variable | BrS Group | Control Group | p Value |
|---|---|---|---|
| Age at defibrillator implantation (yrs) | 48 ± 12 | 59 ± 13 | 0.0003 |
| Men | 38 (93%) | 33 (80%) | 0.08 |
| Follow-up duration (mo) | 0.48 | ||
| Median | 76 | 67 | |
| Interquartile range | 51–98 | 48–87 | |
| Previous cardiac arrest | 9 (22%) | ||
| Syncope | 15 (37%) | ||
| Family history of sudden cardiac death <45 yrs old | 3 (7%) | ||
| Patients with drug test (n) | 26 (63%) | ||
| Patients with positive drug test | 25/26 (96%) | ||
| Patients with electrophysiologic study | 35 (85%) | ||
| Patients with inducible ventricular fibrillation at electrophysiologic study | 33/35 (94%) | ||
| Indication for implantable cardioverter-defibrillator implantation | |||
| Previous cardiac arrest | 9 (22%) | ||
| Syncope and positive electrophysiologic study findings | 15 (37%) | ||
| Family history and positive electrophysiologic study findings | 2 (5%) | ||
| Positive electrophysiologic study findings | 15 (37%) |
| Structural Heart Disease | n (%) |
|---|---|
| Ischemic heart disease | 14 (34) |
| Hypertrophic cardiomyopathy | 12 (29) |
| Dilated hypertrophic cardiomyopathy | 2 (5) |
| Dilated cardiomyopathy | 7 (17) |
| Arrhythmogenic right ventricular cardiomyopathy | 2 (5) |
| Cardiac sarcoidosis | 3 (7) |
| Valvular heart disease | 1 (2) |
In the BrS group, overall, 15 patients (37%) experienced 21 adverse events after ICD implantation ( Table 3 ). Ten patients (24%) received ≥1 episode of inappropriate ICD shock because of atrial tachyarrhythmias (atrial fibrillation or supraventricular tachycardia) or T-wave sensing. A total of 8 patients (20%) experienced 11 device-related complications. The mean interval from implantation to the occurrence of a complication was 64 ± 40 months. The most frequent complication was lead fracture (4 patients) occurring at an average age of 47 ± 4 years. Device infection occurred in 3 patients and required replacement of the generator and lead, with administration of antibiotics. One patient developed symptomatic subclavian vein thrombosis 18 months after implantation that was successfully treated by anticoagulation. In 2 patients, lead perforation resulted in cardiac tamponade. It occurred on the day of device implantation in 1 patient. The patient was successfully treated with percutaneous pericardiocentesis. One patient experienced lead dislodgement requiring lead replacement 5 months after implantation.
| Variable | BrS Group | Control Group |
|---|---|---|
| Implantable cardioverter-defibrillator therapy | 15 (37%) | 21 (50%) |
| Appropriate | 5 (12%) | 14 (34%) |
| Inappropriate | 10 (24%) | 7 (17%) |
| Device-related complications | 8 (20%) | 5 (12%) |
| Lead dislodgement | 1 (2%) | 1 (2%) |
| Lead fracture | 4 (10%) | 2 (5%) |
| Device infection | 3 (7%) | 2 (5%) |
| Symptomatic subclavian vein thrombosis | 1 (2%) | 0 (0%) |
| Cardiac tamponade | 2 (5%) | 0 (0%) |
| Any complication | 15 (37%) | 11 (27%) |
Three patients (7%) experienced 2 major complications. One patient developed lead fracture 39 months after implantation that was treated with new lead placement without extraction of the fractured lead. Device infection ensued 13 months later, which required replacement of the generator and lead assembly. In another patient, lead fracture occurred 116 months after implantation. A new lead was placed without extraction of the fractured lead. One month later, lead perforation resulted in cardiac tamponade, necessitating surgical repair. In another patient, lead dislodgment occurred 5 months after implantation. It was repositioned. At 56 months after repositioning, the lead fractured and was treated with placement of a new lead without extraction of the fractured lead.
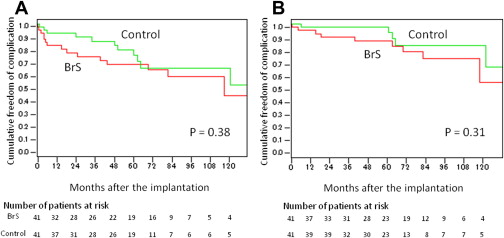
The Kaplan-Meier survival curves ( Figure 1 ) showed that 82.7% (95% confidential interval [CI] 71.0% to 94.3%), 74.2% (95% CI 60.3% to 88.1%), 68.1% (95% CI 53.0% to 83.2%), and 58.8% (95% CI 40.9% to 76.6%) of the patients were free from any complication at 1, 3, 5, and 7 years after device implantation. Excluding inappropriate shocks, 95.1% (95% CI 88.4% to 100.0%), 89.6% (95% CI 79.8% to 99.3%), 86.6% (95% CI 75.6% to 98.0%), and 73.3% (95% CI 56.4% to 90.1%) of patients were free from device-related complications at 1, 3, 5, and 7 years, respectively ( Figure 1 ).
In addition, 2 patients (5%) with asymptomatic BrS required psychiatric assistance during the follow-up period. Both patients came to the emergency room frequently for several months after implantation; however, the psychiatric problem was finally controlled without any medication.
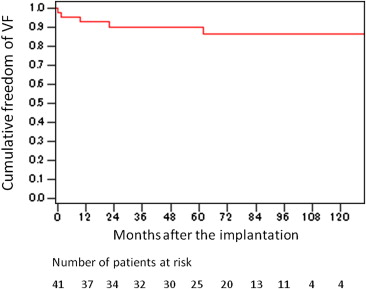
During a median follow-up of 76 months (interquartile range 51 to 98), 5 patients had appropriate ICD shocks for VF. All had previously been resuscitated from cardiac arrest or syncope. The Kaplan-Meier survival curves ( Figure 2 ) showed that 92.6%, 89.8%, 89.9%, and 86.1% were free of lethal arrhythmias at 1, 3, 5, and 7 years, respectively. One patient died from a noncardiac cause 84 months after implantation. Arrhythmic events had not been detected during the follow-up period in this patient.
In the control group, lead fracture and device infection was observed 92 ± 28 and 33 ± 27 months after implantation, respectively, in 2 patients each ( Table 3 ). Although appropriate shocks were delivered more frequently in the control group, the complication rates were similar in the 2 groups ( Figure 1 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


