Chapter 42
Local Complications
Graft Infection
Martin R. Back
The use of prosthetic biomaterials (polymer grafts, metallic stents, endovascular stent-grafts) implanted endoluminally or during open reconstruction within the arterial or venous circulation has permitted palliation of a large number of disabling or potentially fatal vascular conditions. The overall incidence of infection involving vascular prostheses is relatively low because of routine antibiotic prophylaxis before surgical procedures, refinements in sterilization and packaging of devices, and careful adherence to aseptic procedural and surgical technique.
When infection does occur, detection and definitive therapy of the vascular prosthesis are often delayed, with potentially catastrophic consequences. Infection involving a vascular prosthesis is difficult to eradicate. If it is not recognized or treated promptly, implant failure will occur as a result of sepsis, hemorrhage, or thrombosis with end-organ ischemia.1–6
The clinical manifestations of prosthetic vascular infection vary depending on the anatomic location and the virulence of the pathogen.1,3,5,7–9 The resulting clinical spectrum of graft infection can allow surgeons to use a patient-specific treatment approach. However, widely varying treatment approaches, usually encompassing small patient cohorts, have resulted in reports of variable outcomes in the literature, making selection of optimal therapy difficult.
In general, surgical therapy is always required, often coupled with excision of the prosthesis, because antibiotics alone are insufficient to eradicate an established infectious process. Adherence to specific criteria in selecting an appropriate treatment care plan is imperative and is influenced by the clinical findings, anatomic location, time since initial implantation, type of graft/device material, extent of infection, virulence of infecting organism, and underlying patient comorbid conditions.
Keys to a successful outcome include use of accurate diagnostics to identify the infecting organism and extent of graft infection, administration of culture-specific antibiotic therapy, and well-planned surgical intervention(s) to excise or replace the infected graft and sterilize the local perigraft tissues. Appropriate management may involve graft excision alone, graft preservation within the implant wound, in situ graft replacement, or graft excision in conjunction with extra-anatomic bypass grafting.
Improved results have been reported in the past 20 years after both graft excision coupled with extra-anatomic bypass and in situ replacement procedures.10–18 Because most patients with late manifestations have a low-virulence graft infection, in situ replacement therapy with autogenous venous conduits, cryopreserved allografts, or antibiotic-impregnated prostheses to replace the infected grafts has evolved to be a preferred treatment strategy.*
Regardless of the technique used to eradicate the graft infection, success is measured by patient survival, freedom from recurrent infection, patency of the revascularization, and avoidance of major morbidity and amputation. Even when treatment is successful, the morbidity associated with vascular graft infections is considerable, with outcomes often worse than the natural history of the vascular condition that led to graft implantation.
Incidence
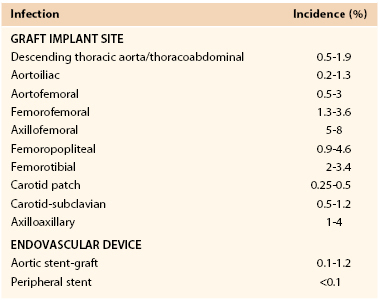
The reported incidence of infection involving a vascular prosthesis varies; infection occurs after 0.2% to 5% of operations and is influenced by the implant site, indication for the intervention, underlying disease, and host defense mechanisms (Table 42-1).† Vascular surgeons now realize that the potential for graft infection extends well beyond the perioperative period. Aortic graft infections can develop months to years after implantation, and thus the long-term incidence is higher. A population-based study from the Mayo Clinic estimated that during a 10-year period after prosthetic grafting of the aorta, the incidence of infection was 5%.34 Graft infection occurs much less frequently than wound infection, with the incidence of early (<30-day) graft infection being in the range of 1% of procedures. Infection is more likely to involve prosthetic grafts implanted during an emergency procedure (e.g., ruptured abdominal aortic aneurysm [AAA], acute arterial ischemia) and to occur when the prosthesis is anastomosed to the femoral artery or placed in a subcutaneous tunnel (e.g., axillofemoral or cross-femoral bypass). In a Canadian prospective multicenter trial of nonruptured AAA open repair, the incidence of graft infection was 0.2%,23 similar to that reported after endovascular stent-graft AAA repair.31,35–37 Infection can also develop after endoluminal stent deployment, but the incidence appears to be extremely low (<0.1%).
Classification
Prosthetic graft infections can be classified according to time of appearance after implantation, relationship to postoperative wound infection (Szilagyi’s classification), and the extent of graft involvement (Bunt’s classification) (Box 42-1).
Early (<4 months after graft implantation) infections correlate with Szilagyi grade III wound infections that involve vascular prostheses.33 These infections are caused by virulent hospital-acquired bacteria and are associated with sepsis, as evidenced by fever, leukocytosis, bacteremia, and advanced wound infection. There is evidence that even Szilagyi grade I and II wound infections increase the likelihood of a late-appearing graft infection. Late infections are the result of graft colonization by low-virulence organisms such as Staphylococcus epidermidis or, infrequently, Candida species.* The low titer of microorganisms on graft surfaces produces an indolent infection without signs of sepsis, and cultures of perigraft fluid or tissue may yield no growth.
Bunt2 proposed using standardized terminology to reflect the spectrum of graft infections and allow comparison of treatment outcomes. Categories of this terminology include perigraft infection (P0, P1, P2, and P3), graft-enteric erosion (GEE), graft-enteric fistula (GEF) (see Chapter 43), and aortic stump infection.
Most early graft infections occur after patients have been discharged from the hospital, typically within 1 to 3 months, and involve extracavitary grafts. Cavitary (i.e., aortic) graft infections are manifested as late (>4 months) infections with a mean time to appearance of more than 40 months.6,10,39,40 Both early and late infections can be accompanied by either total (P0, P1) or partial (P2, P3) graft involvement.
Pathogenesis
Etiology
The presence of a foreign body potentiates the infectivity of bacteria. In 1957, Elek and Conen41 demonstrated that a single braided silk suture significantly reduces the inoculum of staphylococci required to produce a local infection. The risk of foreign body infection is enhanced in the presence of a larger inoculum, more virulent bacterial strains, depressed host immune function, and invasion of sites more remote from host defenses.
Cellular and Biomolecular Events
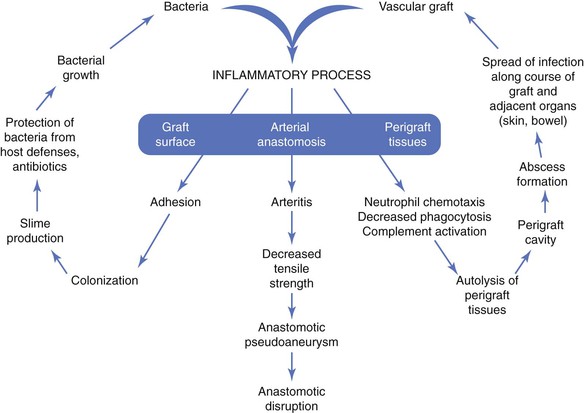
The pathogenesis of biomaterial-associated infection involves the following fundamental steps: (1) adhesion of bacteria to graft or stent surfaces, (2) formation of microcolonies within a bacterial biofilm, (3) activation of host defenses (neutrophil chemotaxis, complement activation), and (4) an inflammatory response involving perigraft tissues and the graft-artery anastomoses (Fig. 42-1).
The initiating event is adherence of bacteria to the biomaterial surface, followed by colonization and development of a bacteria-laden biofilm that resists host defenses and penetration of antibiotics. Both graft and bacterial characteristics influence the likelihood of colonization. Bacterial adherence to polyester grafts is 10 to 100 times greater than adherence to polytetrafluoroethylene (PTFE) grafts. Gram-positive bacteria, such as staphylococci, produce an extracellular glycocalyx, or mucin, that promotes adherence to biomaterials in greater numbers than are seen with gram-negative bacteria. The increased adhesion of staphylococci to biomaterials is due to specific capsular adhesions that mediate attachment and colonization of microorganisms.
The vascular prosthesis and adherent bacteria act together as a coinflammatory stimulus to activate the immune system, in particular, inflammatory cytokines. The local inflammatory response after implantation serves to establish connective tissue ingrowth (“incorporation”) to the outer surface of the graft material. This healing process can be impaired by early perigraft seroma, lymphocele, or hematoma formation, which increases the risk for bacterial adherence and colonization. The inflammatory response to an implanted prosthetic graft also creates an unfavorable environment characterized by local ischemia and an acidic pH that is potentially conducive to bacterial colonization. Local disruption of the fine balance between proinflammatory and antiinflammatory mediators may lead to excess production of matrix metalloproteinases (MMPs) by tumor necrosis factor–stimulated macrophages.42 Excessive degradation of secreted extracellular matrix and angiogenic growth factors by matrix metalloproteinases may hinder optimal graft healing by restricting capillary ingrowth, tissue incorporation, and potential luminal endothelialization. Lack of perigraft ingrowth and vascularity also favors greater exposure of the implanted biomaterial to bacteria and sequestration within graft pores/interstices away from activated phagocytic cells.
Neutrophil function can also be directly impaired in the presence of biomaterials. Decreased neutrophil opsonic, phagocytic, and bactericidal activity against Staphylococcus aureus has been observed in PTFE tissue cages implanted subcutaneously in guinea pigs.43 As local tissue autolysis occurs, the infectious process can spread along the length of the graft and into adjacent structures (e.g., adjacent artery, skin, bowel). The pathobiology is manifested clinically as a spectrum of signs, including graft sepsis, localized perigraft abscess, anastomotic pseudoaneurysm, graft-cutaneous sinus tract, or GEE/GEF (e.g., secondary aortoduodenal fistula). Frequently, the only features of a low-grade, late graft infection are the absence of graft incorporation into surrounding tissue and perigraft fluid containing large numbers of white blood cells (WBCs).
Clinical Sources of Infection
Exposure of a vascular prosthesis to microorganisms (bacteria or fungi) can result in clinical infection by any of four mechanisms: perioperative contamination via the surgical wound, seeding of the biomaterial by bacteremia, mechanical erosion into the bowel or genitourinary tract or through the skin, and involvement in a contiguous infectious process. Underlying impairment of host defenses can further increase the risk for infection.
Perioperative Contamination
Skin and lymph nodes are a major reservoir of bacteria. Biomaterial surfaces can contact microorganisms (1) by a direct route during implantation, (2) through the surgical wound (in the event of a healing complication), or (3) by hematogenous or lymphatic sources arising from remote sites of infection (e.g., urinary tract infection, pedal fungal infection, pneumonia, venous or arterial catheter sepsis, endocarditis, and ischemic foot lesions). Important potential sources of direct graft contamination include breaks in aseptic operative technique, such as contact with the patient’s endogenous flora harbored within sweat glands, lymph nodes, diseased arterial walls (e.g., atherosclerotic plaque or aneurysm thrombus), disrupted lymphatics, and intestinal bag effluents, and injury to or opening of the gastrointestinal or genitourinary tract.
If the surgical wound does not develop a fibrin seal or heal promptly after surgery, the underlying vascular prosthesis is susceptible to colonization from any superficial wound complications (e.g., cellulitis, dermal necrosis, lymphocele). Wounds with persistent drainage indicate the presence of ischemia or tissue injury that, if complicated by superficial infection, can extend to deeper tissue and involve the prosthesis. Diseased arterial walls and reoperative wounds are an unappreciated source of bacteria, with microbiologic culture recovering pathogenic strains of staphylococci in 10% to 20% of cases.1 Bacteria can be harbored in the scar tissue or lymphoceles of healed wounds and can contact prosthetic grafts undergoing revision or replacement for thrombosis or anastomotic aneurysm. Culture of explanted graft material from such procedures has isolated microorganisms, typically S. epidermidis, from 50% to 70% of thrombosed grafts and from more than 80% of grafts associated with anastomotic aneurysms.38
Bacteremia
Bacterial seeding of the prosthesis via a hematogenous route is an uncommon but important mechanism of graft and stent infection. Experimentally, intravenous infusion of 107 colony-forming units of S. aureus produces a clinical graft infection in nearly 100% of animals if administered within days of implantation.44 Thus, bacteremia arising from infected intravascular catheters, urinary tract infection, or other remote tissue infections (e.g., pneumonia, infected foot ulcer) increases the risk of graft infection and occurs not infrequently during the postoperative period in elderly patients undergoing vascular surgery.
Parenteral antibiotic therapy has been shown experimentally to significantly decrease the risk of graft colonization from bacteremia and is the rationale for both antibiotic prophylaxis and culture-specific antibiotic therapy in the patient with a known site of infection. As the prosthesis heals and becomes incorporated into surrounding tissue, susceptibility to bacteremic colonization decreases, but vulnerability has been documented more than 1 year after implantation, with infection developing as the result of dental and gastrointestinal diagnostic procedures. Transient bacteremia, in conjunction with altered immune status, may account for graft infections occurring years after the original operation. It is also possible for a low-grade graft infection to become secondarily infected by a more virulent organism. For example, Escherichia coli urosepsis might inoculate an unincorporated graft that already has a biofilm infection with S. epidermidis, thereby converting a low-grade infection to a more virulent graft infection.
Mechanical Erosion
Erosion of a prosthetic graft through the skin or into the gastrointestinal or genitourinary tract results in a perigraft infection that can spread along the length of the graft. GEE/GEF can develop as a result of pulsatile movement of an aortic graft against adjacent bowel, most commonly without adequate intervening retroperitoneal soft tissue. Enteric erosion may involve the graft body or anastomotic sites with intact suture lines or pseudoaneurysm formation. A low-grade underlying graft infection has been found in a fraction of cases (confirmed by operative findings and recovery of staphylococcal species) and may provide an additional inflammatory stimulus for bowel adhesion.45 The reported incidence of GEE/GEF after prosthetic aortic grafting is 0.4% to 2%. Graft erosion through intact skin is most commonly the result of a chronic, low-grade infection caused by S. epidermidis (see Chapter 43).
Involvement by a Contiguous Infectious Process
Prosthetic grafts can become colonized as a result of an adjacent infection. The most common clinical scenarios are an aortofemoral graft limb infection associated with diverticulitis and a peripheral graft infection secondary to an infected lymphocele. Frequently, the graft segment adjacent to the contiguous bowel or soft tissue infection may be involved. Initial treatment should be directed at drainage of the perigraft abscess and correction of the bowel abnormality if present.
Risk Factors
Multiple perioperative and patient-related factors can increase the risk for development of a vascular graft infection (Box 42-2). In addition to the contributing perioperative factors just discussed, the risk of infection is increased with emergency, extended-length, and reoperative46 reconstructions. A prolonged preoperative hospital stay allows conversion of normal skin flora to hospital-acquired strains that may be resistant to routinely administered antibiotics. Early graft infections are usually the result of wound complications, unplanned reoperation for hematoma or lymphocele, concomitant remote infection, and impaired immunocompetence. Patients with late-appearing graft infections often have a history of multiple operations for thrombosis or anastomotic aneurysm of previously placed prosthetic bypasses.
Impaired Host Defenses
Impaired host defenses from underlying systemic conditions can also predispose patients to prosthetic graft infection.47 The altered immune function associated with malnutrition, malignancy, lymphoproliferative disorders, autoimmune diseases, and drug administration (e.g., corticosteroids, antineoplastic agents, antirejection regimens) may potentiate graft infection with lower numbers of contaminating bacteria.
Although the concept is controversial, diabetic patients appear to have increased susceptibility to infection. Mean plasma glucose levels in diabetic patients before the development of infection and the prevalence of subsequent infections have been tightly correlated in clinical practice. At the cellular level, neutrophil chemotactic and intracellular bactericidal mechanisms are diminished in diabetic patients. Opsonization of S. aureus and E. coli is impaired in such patients. Hyperglycemia in diabetic patients also decreases adherence of neutrophils to foreign bodies.
In addition to the frequent hematogenous introduction of bacteria associated with repeated access to hemodialysis catheters, grafts, and fistulae, patients with chronic renal insufficiency also have higher susceptibility to infection as a result of immune suppression caused by uremia. The depressed neutrophil function in uremia appears to be multifactorial. Neutrophil chemotaxis is inhibited by an unknown agent in uremic serum that specifically blocks the synthesis of chemotactic factor. However, neutrophils from uremic patients placed in normal plasma also exhibit impaired chemotaxis. Adherence, phagocytosis, and bacterial killing by neutrophils and lymphocytes are also diminished in patients with acute or chronic renal failure.
Bacteriology
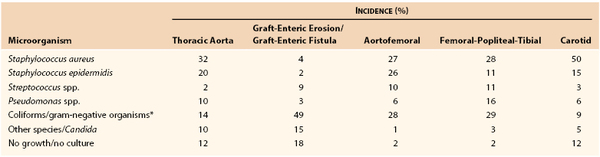
Although virtually any microorganism can infect a vascular prosthesis, S. aureus is the most prevalent pathogen and accounts for one fourth to one half of infections, depending on the implant site (Table 42-2). Graft infections with S. epidermidis or gram-negative bacteria have increased in frequency. This change in the microbiology of graft infection is the result of reporting of both early- and late-appearing graft infections, including aortic graft infections associated with GEE/GEF. Coagulase-negative staphylococci are present in normal skin flora but have the ability to adhere to and colonize biomaterials, where growth occurs within a biofilm on the surface of prostheses. Surgeons have also become aware of microbiologic sampling errors in late infections because of low numbers of bacteria present within the graft surface biofilm and their slow growth.48 Graft infections associated with negative culture results are caused by S. epidermidis or other coagulase-negative staphylococci and, on occasion, by Candida species. Infection with gram-negative bacteria such as E. coli and Pseudomonas, Klebsiella, Enterobacter, Serratia, and Proteus species can be particularly virulent. The incidence of anastomotic dehiscence and arterial rupture is high because of the ability of the organisms to produce destructive endotoxins (e.g., elastase and alkaline protease) that compromise the structural integrity of the vessel wall. Fungal (e.g., Candida and Aspergillus species) and mycobacterial (tuberculous) infections of grafts are rare, and most patients with such infections either are severely immunosuppressed or have an established fungal or opportunistic infection elsewhere.
Plasmid-mediated genetic mutations have afforded S. aureus resistance against penicillin, β-lactams, and other antibiotics (aminoglycosides, erythromycin, tetracycline). Nosocomial and community-acquired infections caused by methicillin-resistant S. aureus (MRSA) have rapidly increased in prevalence over the past decade. Although estimates in the general population have shown MRSA skin colonization (nares and wounds) in less than 2% of individuals, much higher prevalence is found in residents of long-term care facilities (23% to 49%).49–51 A study involving more than 13,000 surgical patients admitted in a University hospital in Switzerland found that the overall incidence of MRSA carriers was 4%, but of those carriers, 64% were newly identified. Previous hospitalization, age greater than 75 years and recent antibiotic treatment were each prognostic for unsuspected MRSA carriage.52 The combination of increased prevalence, harboring in high-risk populations, multiple staphylococcal virulence factors, and rapidly evolving antibiotic resistance mechanisms makes emergence of MRSA a daunting medical challenge. British reports have documented that MRSA is the most common pathogen involved in vascular wound and graft infections and that it is associated with higher morbidity and mortality rates than infection with other microbes.53,54 Our institution has witnessed the prevalence of MRSA arterial graft infections increase fourfold over the past 20 years (from 11% before 2000 to 49% after 2000), with more than half of early extracavitary graft infections being the result of MRSA. Early mortality, limb loss, and infection recurrence rates have not been appreciably higher for MRSA in our experience. However, the future possibility of a higher overall incidence of graft or vascular device infections caused by more prevalent MRSA colonization in the population is more concerning.
Prevention
Prevention of graft infection is imperative, and the surgical team must be cognizant of preoperative, operative, and postoperative prophylactic measures.
Principles
Vascular infections can be minimized if the following principles are applied:
Meticulous attention to sterile technique is imperative to avoid bacterial contact of vascular devices during implant procedures, especially emergency or prolonged reconstructive procedures. Careful handling of tissues, hemostatic technique to prevent hematoma formation, and closure of groin incisions in multiple layers to eliminate dead space are important technical measures that decrease wound complications and the risk of infection. Skin reapproximation without tension minimizes the development of dermal ischemia and wound edge necrosis.
Topical Antibiotics
The addition of topical antibiotics (e.g., bacitracin or cefazolin) to irrigating solutions allows soaking of grafts before implantation and cleansing of wounds before closure and may contribute to decreased wound infection rates. Randomized clinical trials using rifampin-soaked (1 mg/mL) gelatin-impregnated polyester aortofemoral grafts reported significantly reduced groin wound infection rates (4.4% without rifampin versus 2.7% with rifampin), although the rates of subsequent graft infections were similar (0.6% versus 0.3%).55,56 All graft infections were caused by S. aureus in this study. On the basis of the absence of a longer-term benefit, routine rifampin treatment of polyester grafts for primary aortic reconstruction cannot be currently recommended.
Prophylactic Antibiotics
Prophylactic antibiotics should be infused before skin incision and at regular intervals during long procedures to maintain tissue drug levels above the minimal bactericidal concentration for expected pathogens (Box 42-3). Additional dosing may be needed during the operation, depending on the rate of drug elimination and volume of distribution, with larger or more frequent dosing necessary during prolonged (>4-hour) procedures or excessive changes in blood volume, fluid administration, or renal blood flow during the procedure. Prophylaxis should also be instituted before percutaneous puncture of existing prosthetic grafts (e.g., peripheral or coronary arteriography accessed through existing femoral grafts) or before selected endovascular interventions involving stent/stent-graft implantation. Culture-specific antibiotics should be prescribed for patients undergoing vascular graft implantation who have coexisting infections of the leg or another remote site.
At some vascular centers, prophylactic antibiotics are continued for 2 to 3 days in patients deemed to be at high risk for infection from bacteremia, prolonged preprocedure hospitalization, or high (>10%) institutional wound infection rates. Because of the increasing risk of MRSA-related wound complications in these patients, our group advocates prophylactic use of daptomycin (4 mg/kg before and after surgery) as an alternative to vancomycin or cefazolin when prosthetic arterial grafts are implanted.
After implantation of a prosthetic graft, patients should be informed of the potential risk for graft colonization and infection from transient bacteremia, especially after interventional procedures such as dental work, colonoscopy, and cystoscopy. Antibiotic prophylaxis is recommended if these procedures are performed within 3 months of the vascular operation. Amoxicillin 2 g orally 1 hour before the procedure can be used; for the patient with penicillin allergy, clindamycin 600 mg orally 1 hour before the procedure is an alternative.
Diagnosis
Prompt diagnosis and treatment of prosthetic graft infections are essential to avoid major complications (e.g., sepsis and hemorrhage) and death. Clinical manifestations are varied and may be subtle, particularly when associated with cavitary graft infections. Operative exploration is the most accurate method for confirming infection and may be necessary when clinical suspicion of GEE/GEF exists (see Chapter 43). In equivocal cases, the vascular surgeon must prove that a graft infection is not present. The urgency of diagnostic evaluation depends on the clinical findings and the status of the patient.
Clinical Manifestations
Vascular surgeons should maintain a low threshold for proceeding with additional diagnostic testing when any symptom or sign suggests graft infection. In aortic grafts confined to the abdomen, unexplained sepsis, ileus, or abdominal distention might be the only clinical sign. If infection involves an extracavitary graft (i.e., in a limb, the groin, or the neck region), the initial sign of infection is usually overlying inflammation/cellulitis, cutaneous draining sinus tract, or anastomotic pseudoaneurysm. Any patient with gastrointestinal bleeding and an aortic graft should be presumed to have graft infection and GEE/GEF until either another source of bleeding is conclusively identified on endoscopy or no graft-bowel communication is verified at surgery.
In patients with vague suggestive symptoms and ultrasound or computed tomography (CT) evidence of perigraft fluid, careful review of the operative history and surgical notes may furnish clues that further support the diagnosis of graft infection and provide the rationale for invasive diagnostic testing. The patient should also be queried about recent medical illnesses that may have resulted in hematogenous or lymphatic seeding of the graft with bacteria. Early graft infections with S. aureus or other gram-negative bacteria typically manifest within weeks of the procedure as fever, leukocytosis, wound complications, and perigraft purulent drainage. Bacteremia is a sign of an advanced graft infection associated with arterial wall or mural thrombus infection or the secondary development of endocarditis. Patients with grafts infected by S. epidermidis are typically seen months to years after graft implantation with graft-healing complications (e.g., anastomotic aneurysm, perigraft fluid cavity, or graft-cutaneous sinus tract). Systemic signs of sepsis (e.g., fever, leukocytosis, and bacteremia) are frequently absent.
The clinician should carefully examine the site or sites of graft implantation for any signs of inflammation. Surgical incisions should be carefully inspected for erythema and draining sinuses. Masses near anastomotic sites can represent perigraft abscesses or anastomotic pseudoaneurysms. The extremities should be examined for signs of septic embolization (i.e., a cluster of petechiae downstream from the infected graft). Other sources of infection, such as infected foot lesions, osteomyelitis, and infected urinary calculi, should be sought because these conditions can predispose to hematogenous bacterial seeding and graft colonization.
An elevated WBC count with a left-shifted differential count and an increased erythrocyte sedimentation rate are common but nonspecific findings in patients with graft infection and fever. Routine laboratory testing should also include urinalysis, blood culture, and cultures of other clinical sites of infection, such as foot ulcers and surgical wound drainage. Positive blood culture results are uncommon (<5%) but, when present, indicate an advanced graft infection or virulent organisms (or both). All laboratory test results may be normal in patients with late-appearing perigraft infections with S. epidermidis.
Imaging Studies
Vascular imaging is essential for the diagnosis and treatment of graft infection and its sequelae. Anatomic signs of graft infection, such as perigraft abscess, anastomotic aneurysm, and GEE/GEF, can be accurately identified (with >90% sensitivity) thorugh a combination of ultrasonography, CT, magnetic resonance imaging (MRI), arteriography, and endoscopy. Functional radionuclide imaging (gallium Ga 67 citrate, indium In 111–labeled leukocytes, technetium Tc 99m hexametazime–labeled leukocytes) can confirm the presence of a clinically suspected graft infection when anatomic signs of perigraft infection are equivocal.
The combination of anatomic and functional vascular imaging techniques is highly accurate (sensitivity of 80% to 100%, specificity of 50% to 90%) in confirming the presence of infection, planning management, and assessing operative sites for residual or recurrent infection. Arteriography is used to develop an operative strategy for revascularization in the presence of distal ischemia, occlusive disease, or graft thrombosis. CT angiography (CTA) and three-dimensional vessel reconstructions provide improved resolution that may replace planning arteriography.
Imaging studies are performed (1) to identify the extent of infection and perigraft inflammation as well as safe routes of operative exposure and placement of vascular clamps and (2) to minimize the likelihood of injury or organ/limb ischemia secondary to anatomic anomalies or concomitant occlusive disease. Each imaging technique has unique diagnostic applications, and the extent of testing necessary is specific to the individual patient.
Contrast-Enhanced Computed Tomography and Computed Tomographic Angiography
CT is the preferred initial imaging technique for suspected aortofemoral, abdominal, or thoracic aorta and major cavitary branch vessel (P0, P2) graft infections and for extracavitary graft infections involving the neck, torso, and proximal part of the limbs (P1, P3). It should be performed with and without intravenous contrast and oral contrast agents (to detail the relationship of adjacent enteric structures) used only in patients in whom GEE/GEF is suspected. The exact location and extent of polyester and PTFE grafts can best be seen on initial non–contrast-enhanced imaging. Review of delayed arterial-phase images or the “bone” preset window for early arterial-phase imaging best separates the detail of prosthetic graft, arterial, and venous structures from perigraft tissues and aids in image interpretation.
Diagnostic criteria consistent with infection include the loss of normal tissue planes (e.g., fat density) of the retroperitoneal or subcutaneous perigraft structures (indicative of inflammation), collections of fluid or gas around the graft (Fig. 42-2), false aneurysm formation, hydronephrosis, and adjacent vertebral or bony osteomyelitis. Any gas in periprosthetic tissues beyond 2 or 3 months after implantation is an abnormal CT finding suggestive of graft infection. CT angiography provides assessment of continuity of the arterial lumen, associated distribution of occlusive disease, and the presence of thrombus at planned clamp sites and may enable operative planning for arterial reconstruction without invasive arteriography.
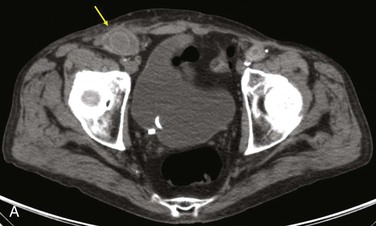
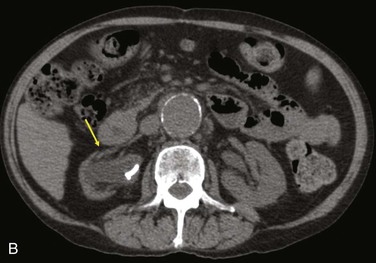
Figure 42-2 Non–contrast-enhanced CT scan showing an infected aortofemoral graft limb (arrow). A, Perigraft fluid and inflammation in the groin. B, Infection-associated ipsilateral hydronephrosis, indwelling ureteral stent, and atrophic kidney.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree