Chapter 47
Local Complications
Lymphatic
Audra A. Duncan
Because of the close anatomic association between fine lymph vessels, lymph nodes, and corresponding arteries and veins, the possibility of lymphatic complications should always be considered when proceeding with vascular operations. Fortunately, the ability of transected or ligated lymphatics to regenerate and re-establish normal lymphatic transport is remarkable. Intraoperative lymphatic injury frequently heals spontaneously and causes minimal or no morbidity. Injury to the lymphatics is, however, a major contributor to the development of lower extremity edema after infrainguinal reconstruction.1–11 Interruption of lymphatic vessels during surgical dissection may also cause a lymphatic fistula 12–17 or lymphocele.17–27 Rarely, injury to the para-aortic or mesenteric lymphatics may result in chylous ascites,22,28–46 thoracic duct injury during cervical, thoracic, or thoracoabdominal aortic reconstruction,31,47–56 or may result in chylothorax after high translumbar aortography.57
This chapter reviews the pathophysiology, diagnosis, and management of the most frequent lymphatic complications after vascular reconstruction and suggests guidelines for prevention.
Post-Bypass Edema
Lower extremity edema occurs in 50% to 100% of patients who undergo open infrainguinal arterial reconstruction for chronic ischemia.2,7 Leg swelling after femoropopliteal or femorotibial bypass becomes evident with dependency, usually when the patient resumes ambulation. Pitting edema generally subsides within 2 to 3 months after reconstruction, although it may be persistent in some patients. Significant lymphedema can impair normal ambulation or delay wound healing despite successful arterial reconstruction.
Etiology and Pathogenesis
Lymphedema develops when the rate of production of protein-rich interstitial fluid exceeds the capacity of the lymphatic system to remove the increased lymph volume. When lymphatic transport fails, post-bypass edema occurs.58 Lymphatic insufficiency has two main causes. First, increased production of interstitial fluid after successful revascularization results in a significant increase in the lymphatic load. Second, the transport capacity of the lymphatic system is reduced because of lymphatic injury and obstruction of deep and superficial lymph channels during dissection in the groin and popliteal space and along the great saphenous vein.
Increased capillary filtration results from elevated arterial pressure after revascularization, alterations in the regulation of microcirculatory flow, and probable endothelial and smooth muscle injury from chronic ischemia.1,7 Eickhoff8 demonstrated that abnormalities in local blood flow regulation normalize in about a week after reconstruction, whereas edema persists much longer in these patients. Although derangement of the microcirculation contributes to post-bypass edema to some degree, Eickhoff’s experiments support the theory that lymphatic obstruction secondary to surgical injury is the most important cause of post-bypass edema.
If the number of functioning major lymph channels decreases to a critical level, lymphedema develops. In one study in which patients underwent lymphangiography after infrainguinal bypass, the average number of patent superficial lymph vessels visualized was reduced to 1.7 per patient compared with the normal average of 9.5.1 In a similar series of 37 patients, edema was not significant when more than 3 intact superficial lymph vessels were visualized on the postoperative lymphangiogram.4 AbuRahma et al9 examined the involvement of the lymphatic system in the pathophysiology of edema formation in patients who underwent femoropopliteal bypass grafting. Edema developed in 29 of the 72 patients (40%). Leg swelling occurred in 85% (17 of 20) of the patients treated by conventional dissection of the femoropopliteal arteries. When careful dissection preserving lymphatics was performed, edema developed in only 2 of 20 patients (10%). Postoperative lymphangiography showed normal anatomy in six of the eight patients without edema, but the anatomy was markedly abnormal in all eight patients with edema who underwent lymphangiography.
Persson et al10 found less edema in patients who needed less dissection during surgery. Significantly less swelling was observed in patients with prosthetic grafts than in those with vein grafts. Patients with above-knee grafts also had less edema than those with below-knee bypasses.10 Studies using albumin clearance in patients with edema after revascularization also supported the idea that edema is mainly lymphatic in origin. A reduction in the plasma albumin level with a concomitant increase in albumin content in the extremity was noted after femoropopliteal bypass.59 The increase in albumin content was three times greater in limbs revascularized by femoropopliteal bypass than in those revascularized with aortoiliac grafts. These data correspond to the clinical observation that edema rarely develops after aortofemoral revascularization. Although venous thrombosis has been proposed as a cause of postoperative leg edema,60,61 studies have demonstrated a low incidence of deep venous thrombosis in patients with post-bypass edema.62,63 In one series, normal venous hemodynamics and morphology were confirmed in 41 of 45 patients with leg edema after arterial bypass.64 The incidence of deep venous thrombosis after femoropopliteal bypass was found to be similar in patients who had edema (7%) and in those who did not (10%).9 Deep venous thrombosis therefore seems to play a minor role in post-bypass edema in most patients.7,58,65
Diagnosis
Mild, partially pitting ankle edema appears on the second or third postoperative day and resolves almost completely with leg elevation and bed rest. Lymphedema is frequently unilateral and involves the dorsum of the foot (“buffalo hump”) and the toes (“squaring”). The differential diagnosis of postoperative limb swelling includes deep venous thrombosis, infection with cellulitis, and compartment syndrome. The history and physical examination can aid in identifying cellulitis or compartment syndrome, and duplex scanning of the deep veins is the test of choice for excluding deep venous thrombosis. If the cause of the edema is still in question, lymphoscintigraphy can confirm the presence of lymphedema (Fig. 47-1).
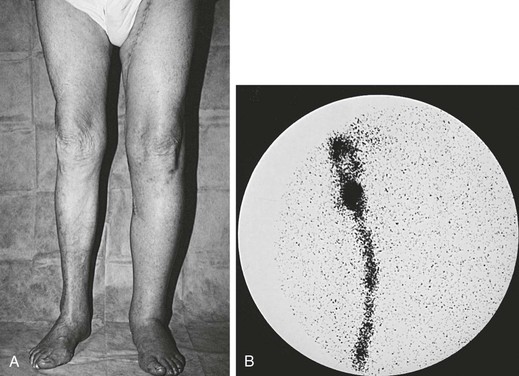
Figure 47-1 A, Edema of the left lower extremity in an 88-year-old man 4 weeks after left femoropopliteal saphenous vein bypass performed for severe chronic ischemia. B, Lymphoscintigraphy confirmed severe lymphedema of the left leg with no visualization of the lymph vessels or inguinal lymph nodes. Lymphatic transport was normal on the right.
Management
Postoperatively, mild edema of the extremity should be treated by frequent elevation of the limb and some restriction of ambulation. Cardiac failure should be treated promptly to help preserve the normal pressure gradient and allow venous return and lymph flow toward the heart. Moderate to severe post-bypass edema is treated with compression wrapping until the incisions have healed, followed by calf-length, 30 to 40 mm Hg compression stockings. For patients with a below-knee, in-situ bypass or any bypass to the distal tibial or pedal arteries, management is individualized to avoid direct compression of the subcutaneous vein graft. Attempts to prevent or limit post-bypass edema pharmacologically with steroids, mannitol, terbutaline, or furosemide have not proved effective10 and are not recommended.
Prevention
Meticulous, lymph-preserving surgical dissection is needed to minimize post-bypass edema.9,58 For infrainguinal bypass, a vertical groin incision slightly lateral to the femoral pulse should be made in an attempt to preserve the patency and integrity of the lymph nodes. The inguinal lymphatics should be retracted medially, and a vertical incision should be made in the femoral sheath to dissect the femoral arteries. Loupe magnification facilitates identification of the lymph nodes and lymph vessels. The lymphatics should be carefully preserved; if they must be divided, they should be ligated or cauterized to avoid leakage of lymph. Attempts should be made to preserve as much lymphatic tissue as possible between the saphenofemoral junction and the femoral artery. A skin bridge should be left between the groin incision and the incision made in the thigh to dissect the more distal portion of the saphenous vein. Multiple short skin incisions to harvest the saphenous vein disrupt fewer superficial lymphatics.9 In theory, endoscopic video-assisted vein harvest may also cause less lymphatic injury and decrease wound-healing problems without compromising vein conduit quality. In a series of 68 lower extremity bypass procedures, only 1 bleeding complication was related to the video-assisted harvest, and 2 seromas developed at the arterial dissection sites.66 The benefit of endoscopic versus open vein harvesting in terms of a reduced rate of lymphatic complications was confirmed in prospective randomized studies,67,68 and such harvesting can result in a reduction in postoperative wound infections.69 Published evidence supports the use of endoscopic vein harvest to decrease infectious wound complications and improve cosmesis, in addition to improving outcomes and decreasing the need for interventions because of graft occlusion.70 Dissection around the popliteal artery should be performed with the same care to avoid lymphatic disruption. The vascular sheath should be opened longitudinally without dissection of the popliteal vein or the posterior tibial nerve in the neurovascular bundle. Fibroadipose tissue, which contains the deep lymphatics in the popliteal fossa, should be left intact.
Lymphatic Fistula
Because of the rich lymphatic network in the femoral triangle, lymphatic fistulae after vascular reconstruction most often occur at the groin. In 4000 vascular operations, Kalman et al16 observed lymphatic fistulae in 45 patients (incidence, 1.1%). The incidence of this complication was similar in other series and ranged from 0.8% to 6.4%.13,71,72
Etiology
Important factors that contribute to lymphatic leakage are failure to ligate or cauterize divided lymphatics and failure to approximate the tissue layers properly at closure. Lymphatic leakage occurs more frequently in older diabetic patients with poor wound healing. Excessive early limb motion, infection of the operated leg or foot, reoperation, and placement of a prosthetic graft to the groin are other possible causes.16
Diagnosis
Persistent leakage of clear or yellow fluid from a groin incision establishes the diagnosis. Lymphoscintigraphy to confirm that the fluid is of lymphatic origin is seldom necessary when the fistula develops within days or a few weeks after the operation. When lymphatic leakage occurs several months or years after vascular reconstruction, lymphoscintigraphy is helpful; in such cases, however, computed tomography (CT), white blood cell scanning, and sometimes fistulography must be performed to exclude infection of an underlying vascular graft. CT is also valuable for the diagnosis of concomitant retroperitoneal lymphatic injury, because a retroperitoneal lymphocele or chylous ascites can be manifested as a lymphatic fistula at the groin.58
Management
Early diagnosis and management of a lymphatic fistula are important to prevent prolonged hospitalization and delayed wound healing. Although infection of an underlying vascular graft was not noted in 1 study of 35 patients with lymphatic leakage, most studies have reported a small but definite risk of deep wound infection from persistent leakage of lymph.13,16 Conservative management is indicated in the first few days and should include local wound care, administration of systemic antibiotics, and bed rest with leg elevation to reduce lymph flow.
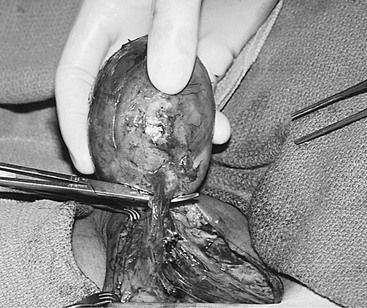
When the fistula continues to produce large volumes despite several days of conservative management, surgical closure in the operating room is the best therapeutic option.13,16 Approximately 30 to 60 minutes preoperatively, 5 mL of isosulfan blue (Lymphazurin; United States Surgical, Norwalk, Conn) dye is injected subcutaneously into the first and third interdigital spaces in the foot (Fig. 47-2).15,58 A sequential compression pump is placed on the affected leg to increase lymphatic and venous drainage. The groin incision is then opened, and the site of the lymphatic injury is apparent by the leakage of blue fluid droplets (Fig. 47-3). The area is oversewn, and the wound is closed in multiple layers. If isosulfan blue is not visualized, the incision is irrigated and closed in layers with interrupted suture. When it is impossible to oversew the damaged tissue, injection of fibrin glue may also be useful.
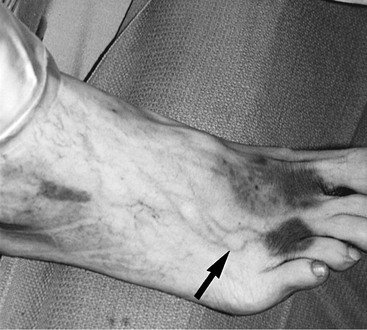
Figure 47-2 Injection of isosulfan blue (Lymphazurin) dye into the first and third interdigital spaces of the foot immediately visualizes the foot lymphatics (arrow) and during surgery helps identify the site of lymphatic injury at the groin.
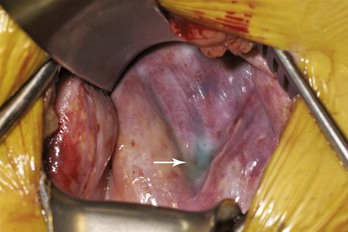
Figure 47-3 Intraoperative photograph of groin re-exploration demonstrating isosulfan blue (Lymphazurin) dye at the base of the incision (arrow). The dye allows localization of the lymphatic injury to facilitate ligation and fistula control.
Recently, the widespread use of vacuum-assisted closure (VAC) therapy has been suggested in the use of lymphocutaneous fistulae.73 By placing VAC devices on 10 patients with either lymphocutaneous fistulae (4 patients) or lymphoceles (6 patients), which converted to fistulae, the authors were able to treat all incisions without recurrence with a median outpatient VAC treatment duration of 16 days. Although this was an early study, it suggests that VAC therapy may be useful in recurrent lymphoceles or difficult to manage wounds.
Lymphocele
A lymphocele is a localized collection of lymph. Early after injury to the lymphatic pathways, the lymph collects between tissue planes. Unless the lymph is reabsorbed spontaneously or drains through a cutaneous fistula, a pseudocapsule develops. In contrast to a seroma, a lymphocele usually has a well-localized connection with one or more of the lymphatic channels. For this reason, lymphoscintigraphy can readily demonstrate a lymphocele (Fig. 47-4).
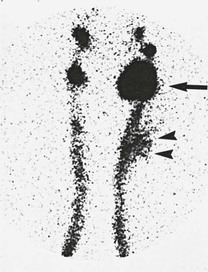
Figure 47-4 Bilateral lower extremity lymphoscintigraphy demonstrating a large left groin lymphocele (arrow) and extravasation of colloid in the left side of the thigh (arrowheads).
Groin Lymphocele
As with lymphatic fistulae, the most frequent site of lymphoceles after vascular reconstruction is the groin. Most lymphoceles develop in the early postoperative period but may appear later. Large lymphoceles cause local discomfort, pain, and leg swelling. Hematoma, seroma, and wound infection should be considered in the differential diagnosis. The presence of a soft, fluid-filled cyst and intermittent drainage of clear lymph through a fistula confirms the diagnosis of lymphocele. Ultrasonography is helpful in distinguishing a solid, dense hematoma from a cystic lymphocele. CT is performed when a lymphocele develops several weeks to months after the operation. CT is helpful in excluding graft infection or identifying a retroperitoneal lymphocele extending to the groin.
Small lymphoceles can be observed because they may reabsorb spontaneously. For enlarging or symptomatic lymphoceles or lymphoceles that lie close to a prosthetic graft, early surgery to reduce the chance of graft infection should be performed. Injection of isosulfan blue into the foot is helpful for identifying the lymphatic channels supplying the lymphocele. The lymphocele is excised, and the lymphatic pedicle is ligated or oversewn (Fig. 47-5). The wound is closed in multiple layers over a small subcutaneous drain. Sclerotherapy, performed more frequently for retroperitoneal lymphoceles, can also be used for groin lymphoceles.
Retroperitoneal Lymphocele
Symptomatic retroperitoneal lymphoceles are rare. In a review of more than 4000 aortic reconstructions, an incidence of 0.1% was reported by Garrett et al.25 In reviewing the literature, 11 well-documented cases of this complication were found after aortic reconstruction.* The number of unreported and asymptomatic cases is undoubtedly higher. Retroperitoneal lymphoceles have been reported more frequently after renal transplantation (incidence of 0.6%-18%).26,74–76 In these patients, however, a lymphocele develops not only because of injury to the recipient pelvic lymphatics but also because of increased lymph production and leakage from the donor kidney.26
Diagnosis
The most common symptoms of retroperitoneal lymphocele are abdominal distention, nausea, and abdominal pain, and the most frequent finding is an abdominal or flank mass. Although signs or symptoms may develop early, in almost half of patients, the lymphocele is discovered a year or several years after the operation.58 Patients with signs or symptoms of a retroperitoneal lymphocele should be examined with CT (Fig. 47-6). In 5 of 11 published cases of retroperitoneal lymphocele, a groin mass was also present.58 Evaluation of these patients showed communication between the groin lymphocele and a retroperitoneal lymphocele. This observation illustrates the importance of CT when a groin mass develops after aortofemoral reconstruction. If infection is suspected, white blood cell scanning should also be performed, unless CT has already confirmed graft infection. Lymphoscintigraphy can be diagnostic of a retroperitoneal lymphocele and should distinguish it from perigraft seroma. Nevertheless, lymphoscintigraphic confirmation of a lymphocele does not rule out graft infection.
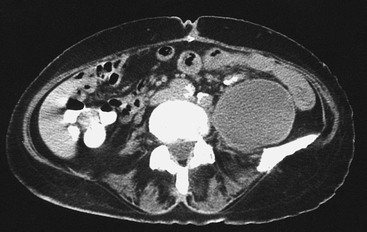
Figure 47-6 CT in a 70-year-old woman reveals a large left retroperitoneal lymphocele 9 months after repair of a thoracoabdominal aortic aneurysm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree