Chapter 45
Local Complications
Graft Thrombosis
David H. Stone, Daniel B. Walsh
Postoperative graft thrombosis remains a significant clinical challenge in contemporary vascular surgical practice. Whether early or delayed, graft thrombosis continues to account for significant morbidity, limb loss, and mortality in patients requiring vascular intervention. Specifically, at 1 year after infrageniculate bypass graft failure, more than 50% of patients will have undergone major amputations.1,2 Among the remaining patients, ischemic pain at rest or ulceration will have developed in 25%, and more than 15% will have died. Underlying risk factors associated with graft failure continue to be the focus of vascular surgical outcomes analyses and regional quality improvement groups alike.3–5
The causes of graft thrombosis are multifactorial and involve patient demographics, risk factors, and comorbid conditions, as well as technical issues associated with arterial reconstruction. Such risk factors and technical aspects of reconstruction have an impact on graft patency from the initial operation through the entire follow-up period. With this in mind, technical precision at initial reconstruction is imperative to achieve an optimal outcome because technical errors account for 4% to 25% of early failure after revascularization.6–8 Furthermore, optimal long-term graft durability remains, in part, predicated on lifetime surveillance, timely re-interventions, and vigilant risk factor modification.9–12 To facilitate intraoperative assessment of the technical adequacy of the reconstruction at the time of surgery, numerous diagnostic tools are available to the surgeon, which we review. Thereafter, the discussion focuses primarily on surgical revascularization, including autogenous and prosthetic conduits and factors associated with their failure. Understanding the etiology and clinical manifestations of graft thrombosis and current experience with available treatment options is crucial for achieving the best and most durable results after initial failure of revascularization.
Prevention: Techniques of Graft Assessment
To ensure optimal patency after revascularization, it is imperative that the surgeon determine the technical adequacy of the reconstruction before leaving the operating room.
Inspection, Palpation, and Measurement of Flow
The most convenient and readily available methods for graft assessment include inspection and palpation of pulses. These processes involve not only inspection of the graft itself for kinks, twists, and stenoses, but also examination of the distal target vessel and of the revascularized tissue, if possible. Intuitive to this process are the surgeon’s expectations. Is the foot pink and perfused? Has capillary refill time been shortened? Is a pulse now palpable in the foot?
The process is facilitated by having the target organ, as much as possible, included in the sterile field and available to the surgeon for intraoperative examination. For example, for aortobifemoral or more distal bypasses, covering the sterilely prepared feet with clear plastic bags permits rapid examination after completion of the bypass. However, inspection and palpation are subjective and thus susceptible to observer bias. After a complex reconstruction, the surgeon’s expectations may cloud the evaluation of capillary refill time in the feet. Calcified arteries secondary to long-standing diabetes may not adequately transmit an improved pulse. The effects of anesthesia combined with concomitant chronic occlusion of runoff arteries may delay the appearance of adequate lower extremity reperfusion. More importantly, false-negative results can occur when a graft has a strong pulse because of distal outflow obstruction.
Measurement of arterial inflow and outflow is often significantly affected by anesthesia. Furthermore, small, seemingly hemodynamically insignificant defects in the graft may also result in failure. Low-flow measurements may accurately predict graft failure, but this finding alone does not localize the specific defect, once discovered. Studies using an ultrasound flowmeter have confirmed the inability of graft flow as an isolated parameter to predict future graft function.13 Measurement of flow by ultrasonically measured transit times has been reported to be sensitive and specific for graft defects.14 Performance of these studies is cumbersome, however, and requires additional adjunctive measures to both localize and identify specific graft defects. Because of these problems and the ease and effectiveness of other techniques, these measures are used less frequently and as perfunctory diagnostic adjuncts.
Arteriography
Since its introduction, intraoperative completion arteriography has been the “gold standard” for anatomic evaluation of the technical adequacy of arterial reconstructions. One appealing feature of arteriography is its ability to assess anatomic arterial outflow—the “runoff.” This is particularly important in the clinical context of preoperative studies that fail to reveal adequate target vessels in diffusely diseased vascular systems. Although completion arteriography is an invasive procedure associated with potential complications because of arterial puncture (intimal injury, dissection), injection (air embolism), use of radiographic contrast agents (renal failure, anaphylaxis), and radiation exposure, the actual observed complication rate has been negligible in large series.15,16
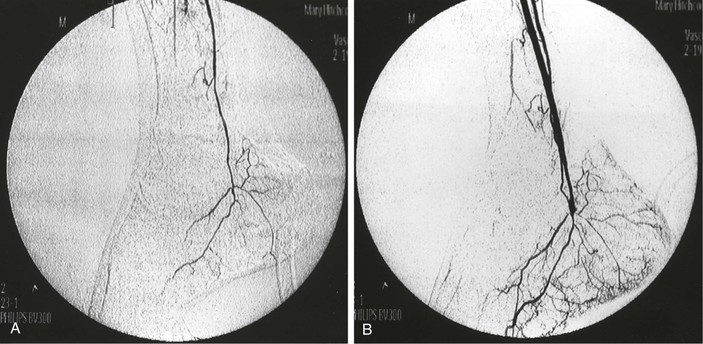
The technique varies according to individual application but generally involves insertion of an 18- to 20-gauge plastic angiocatheter or 5- to 6-Fr sheath into the arterial graft to allow subsequent injection of 10 to 30 mL of radiographic contrast agent. Temporary occlusion of arterial inflow maximizes the concentration of contrast agent without the need for excessively rapid injection. Portable C-arm fluoroscopy units or fixed imaging operating room suites that permit sophisticated imaging after arterial reconstruction are now available in most centers performing vascular care. The presence of such technology in both the operating room and angiography suites will clearly facilitate more routine graft interrogation throughout the follow-up interval when needed (Fig. 45-1).
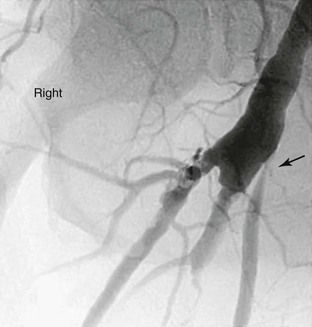
Figure 45-1 Follow-up surveillance angiogram of a femoral-to-popliteal bypass graft with a significant stenosis noted at the proximal anastomosis (arrow). This angiogram was performed after graft duplex ultrasound detected increased velocity at this location.
Several weaknesses are inherent in the technique of arteriography, however. In lower extremity bypass grafts, the proximal anastomosis is frequently not evaluated, which may obviate detection of a proximal technical defect. Air bubbles or overlying structures may lead to false-positive interpretations. A potential source of false-negative results is the use of a single plane of view to analyze a multidimensional target; this method can result in underestimation of the stenosis from a small defect, such as an intimal flap or platelet aggregate.17 Another problem associated with arteriography is the use of potentially nephrotoxic iodinated contrast agents.
To avoid these problems, intra-arterial digital subtraction angiography (DSA) using a portable, axially rotatable imaging device can more easily obtain views from different angles.18 The small amounts of dye and the “cine” nature of modern fluoroscopic units enable visualization of particular areas of the graft, and thereby, allow more accurate interpretation of images. DSA also permits the use of smaller contrast volumes and real-time video replay. DSA is more applicable to localized areas, such as a distal anastomosis and pedal runoff, than to an entire extremity. An entire extremity can be filmed with this technology using repeated injections of small amounts of contrast agent to obtain sequential angiographic images, or the so-called pulse-chase technique, in which the DSA machine is moved along the extremity and “chases” the injected contrast material (Fig. 45-2).
Ultrasonography
The development of ultrasound technology has provided multiple noninvasive modalities for the intraoperative and subsequent longitudinal assessment of arterial reconstructions.
Continuous-Wave Doppler
The simplest and least expensive ultrasound device is continuous-wave Doppler with an 8- to l0-MHz pencil probe. The major advantages of these probes are that they are easy to sterilize with gas and are readily available for intraoperative use. Their small size allows insonation of arteries in areas less accessible to larger probes. With sterile saline used for acoustic coupling, the probe can be passed along the graft or reconstruction site, where localized increases in the audible sound frequency or audible turbulence indicates a potential defect. Patent residual vein branches in in-situ saphenous vein bypasses can be readily identified by locally increased frequency, continuous flow, and flow outside the graft boundary. Successively compressing the graft from the proximal to the distal end while listening for residual proximal flow with a Doppler device can also localize these arteriovenous fistulae.
Use of the audible continuous-wave Doppler technique is subjective and operator dependent. Considerable experience is required for maximum accuracy. The presence of high-frequency sound waves within the graft or at the distal anastomosis is worrisome. However, some investigators have shown that the continuous-wave Doppler device is not highly sensitive.19 Most vascular surgeons use this modality as an easy, available, and inexpensive screening device to guide their use of a more precise evaluation technique. To improve the objectivity of this technique, a fast-Fourier transform spectral analysis can be used to quantify changes in frequency or velocity. A further potential refinement is the use of a high-frequency (20-MHz) pulsed Doppler device contained in a small probe that permits easy access to all operative sites.20 Considerable experience with pulsed Doppler probes is required to achieve accurate results, however, and the technique does not provide the anatomic images that are reassuring to most surgeons considering arterial re-exploration.
B-Mode Ultrasonography
B-mode ultrasonography has been used intraoperatively to obtain anatomic images noninvasively, although it is more commonly used in conjunction with duplex ultrasonography. Initial experimental studies established that its ability to detect small arteriographic defects in patients was comparable to that of arteriography.21 In an evaluation of arterial defects created in dogs, both arteriography and B-mode ultrasonography were nearly 100% specific in excluding arterial defects. However, ultrasonography has significantly greater sensitivity in detecting defects, 92% overall, than serial biplanar arteriography at 70% and portable arteriography at 50%. These techniques have comparable accuracy in detecting stenoses.
B-mode ultrasonography has utility in assessing lower extremity arterial reconstructions. Kresowik et al22 reported that in 106 patients, intraoperative B-mode ultrasonography detected defects in 20% of patients, and that half of these defects were deemed important enough to warrant correction. In follow-up, there were no early graft occlusions in the B-mode group, and no residual defects were discovered with duplex scanning follow-up in the postoperative period.
Intraoperative use of B-mode ultrasonography, however, is not without its problems. Because this modality does not evaluate blood flow, it cannot differentiate fresh thrombus from flowing blood, which has the same echogenicity. Compared with Doppler pencil probes, B-mode ultrasound probes are larger and cannot be sterilized. Thus, their use is more cumbersome. The probes require a sterile covering containing a gel to maintain an appropriate acoustic interface. Significant operator experience is needed to obtain optimal images and make accurate interpretations.
In clinical situations, one difficulty with the technique is determining the significance of the many defects identified, because most do not require repair. The lack of accompanying blood flow information makes this decision more difficult. In one study, B-mode ultrasonography failed to create technically adequate images for evaluation in nearly 25% of patients. Such inadequacy can frequently be encountered during graft interrogation in limited operative fields, and therefore, can confound the probe manipulation required for accurate imaging.23
Duplex Ultrasonography
With the addition of flow-measuring capability to B-mode ultrasonographic technology, duplex scanning brings a more powerful, although more expensive and complex tool, to the operating room. Like B-mode ultrasound probes, duplex scanning probes are large, cannot be sterilized, and require considerable operator skill, not only to obtain accurate images, but also to position the probe over the sample target appropriately so that accurate velocity measurements can be obtained. Duplex color-flow technology provides continuous Doppler signals along the graft and artery at multiple points. Color imaging facilitates identification of areas of higher velocity, albeit at a significant increase in equipment cost.24
Examination of outflow arteries with duplex scanning is less precise than with arteriography, although the information provided is physiologic rather than purely anatomic. Duplex scanning provides an easier mechanism for identifying defects in proximal arterial anastomoses than arteriography does, because contrast-enhanced imaging of the proximal anastomosis from a more distally placed catheter is cumbersome and often difficult.25 In addition, duplex scanning can identify low graft velocities that are undetectable by arteriography. Intraoperative experience with this imaging modality has shown greater sensitivity for detecting technical defects. Early results with intraoperative duplex scanning have demonstrated an association between these defects and suboptimal results in the postoperative period.26,27 It is noteworthy that duplex scanning is unable to access newly placed polytetrafluoroethylene and polyester (Dacron) grafts because the graft walls contain air, which prevents penetration of the ultrasound waves.
In a study from 2002, Rzucidlo et al28 reported intraoperative completion duplex scanning to be a useful tool after the completion of infrageniculate arterial reconstruction. Specifically, the authors documented that a 10-MHz, low-profile transducer could be used successfully to identify compromised grafts with a predilection for early failure. Moreover, it was determined that low end-diastolic velocity was both associated with and predictive of early graft failure. More recently, Scali et al29 validated the utility of intraoperative completion duplex scanning following distal bypass, documenting that end-diastolic velocity (EDV) measurements less than 5 cm/s predicted early graft failure, and more broadly, crural vein graft patency.
Angioscopy
Intraoperative angioscopy has become an attractive technique for evaluation of arterial reconstructions and thorough interrogation of autogenous conduit. Angioscopy requires irrigation with saline accompanied by inflow, and sometimes outflow, occlusion to provide a visually clear image. The presence of any red blood cells can completely obscure accurate visualization of the graft lumen. The use of a specifically designed infusion pump with high- and low-flow rates has greatly facilitated angioscopy techniques.30 As with duplex ultrasonography, experience is required to manipulate both the angioscope and the visual target to obtain adequate and clear visualization.
Angioscopy has been most widely used for inspection of in-situ saphenous vein grafts to ensure complete valve lysis,31 exclude unligated venous branches, and assess the quality of the venous conduit (Fig. 45-3). A 1.4-mm-diameter angioscope is most commonly used at our institution to interrogate lower extremity vein grafts. The angioscope is inserted through an introducer in the most proximal end of the vein or through the most proximal vein branch, which can be deliberately left unligated for this purpose. Saline irrigation is administered through a sheath. Before angioscopy, it is useful to identify and ligate as many venous side branches as possible to optimize distal visualization while minimizing the irrigation required to clear red blood cells. Angioscopy can be used at other sites if blood flow can be temporarily occluded. Such occlusion sometimes necessitates the use of balloon occlusion catheters if proximal control is not surgically accessible. Angioscopy appears to be particularly important in detecting abnormalities within an arm vein conduit that may not be evident by external visual inspection, including important evaluation of venovenostomy anastomoses.32–34
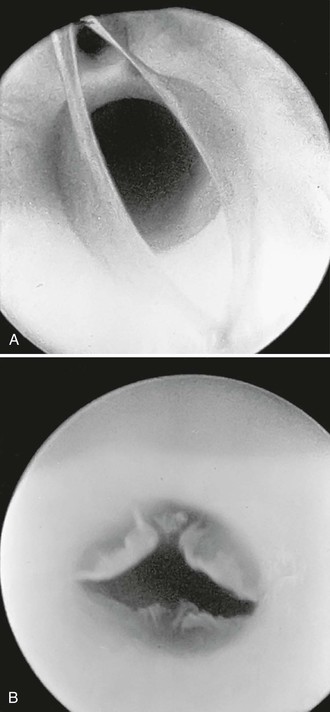
Figure 45-3 A, Photograph of a valve via an angioscope before lysis. B, Photograph of an angioscopic image of a valve after lysis. (B, From McCaughan JJ, Jr, et al: In vitro observations of greater saphenous vein valves during pulsatile and nonpulsatile flow and following lysis. J Vasc Surg 1:356, 1984.)
It should be noted that experimental studies have documented that mild intimal injury may occur with angioscopy. However, such injury occurs only after multiple repeated passages of larger diameter scopes.35 The long-term effects of this mild trauma have not been firmly established, but a few passes of a small-diameter (1.4 mm) angioscope in human vein grafts appear to have no significant late clinical consequences. Several studies have revealed that infusion of irrigation solution can be limited to 500 mL or less in most instances, an amount that has not been associated with complications, especially when planned as a part of the overall fluid administration during the procedure.30,36 In a 2002 study from Lund, Sweden, the authors documented successful application of intraoperative angioscopy in a group of patients who underwent below-knee in-situ saphenous vein graft bypass; they demonstrated its utility in this setting and concluded that angioscopy has an impact on primary graft patency rates, and therefore, reduces the need for subsequent re-intervention. Specifically, this adjunct was useful in identifying persistent saphenous vein branches, incomplete valve destruction, and partially occlusive intraluminal thrombus.37
Intravascular Ultrasonography
More frequently in contemporary practice, intravascular ultrasound (IVUS) has become a useful intraoperative imaging adjunct. This technology is based on a flexible catheter system and generates two-dimensional cross-sectional images through the circumferential rotation of a miniaturized (10-30 MHz) ultrasound crystal at the catheter tip. In experimental studies, IVUS has proved to be accurate in measuring luminal diameter and identifying stenoses caused by atherosclerosis or intimal hyperplasia.38–40 This technology has significant appeal as an imaging modality in the setting of complex aortic procedures, in particular for acute aortic dissection.41–43 Both IVUS and angioscopy were found to be 100% accurate in detecting 2-mm intimal flaps in canine femoral arteries compared with only 60% accuracy for single-plane arteriography.44
Few clinical studies assessing the role of IVUS have established its efficacy and potential role in evaluating the technical adequacy of vascular surgical reconstructions. However, its utility appears to be growing. Although IVUS appears to be useful in the evaluation of lesions appropriate for placement of devices such as bare-metal stents and stent-grafts,45 it is unclear whether IVUS will provide information that is different and useful enough to justify its current cost in comparison to alternative intraoperative techniques. Nevertheless, this real-time, radiation-free, contrast-free, dynamic imaging modality offers significant appeal.
Miscellaneous Modalities
In addition to direct methods of evaluating arterial reconstructions intraoperatively, several indirect methods can measure resistance within the graft or within the outflow bed, which may, in turn, help evaluate the adequacy of revascularization. This is most easily accomplished intraoperatively with a continuous-wave Doppler probe placed over a distal artery while the examiner listens for audible augmentation of the waveform after release of a temporary graft occlusion. Although frequently used intraoperatively, this modality provides little objective quantifiable measure of the adequacy of reconstruction.
A more quantitative assessment can be obtained by measuring distal extremity pressure with a sterile blood pressure cuff during surgery. In patients with more proximal reconstructions and residual outflow abnormalities, ankle pressure may not be maximal immediately after revascularization, but rather may increase only gradually in the postoperative period. Thus, this intraoperative pressure measurement does not provide absolute proof of the success of reconstruction, and therefore, must be interpreted on the basis of the preoperative anatomy of each patient. Other similar modalities, including pulse volume recording (plethysmography), strain-gauge plethysmography, photoplethysmography, and even transcutaneous oxygen tension measurement, can be used intraoperatively as clinical adjuncts to evaluate the restoration of distal blood flow. In each, the surgeon should assess for a significant difference in magnitude with and without graft occlusion.
Outflow resistance can be measured intraoperatively to predict subsequent graft failure in arterial reconstructions. This technique enables calculation of outflow resistance on the basis of the pressure measured while saline is injected into the distal end of a bypass graft at a known rate. Ascer et al46 found that grafts with an outflow resistance of greater than 1.2 resistant units (pressure in mm Hg divided by flow in milliliters per minute) all experienced failure within 30 days. Other groups, however, have not confirmed this observation and have reported long-term patency in grafts with high outflow resistance, especially when using a vein for a conduit.47,48 Like other indirect methods, this technique does not provide anatomic information sufficient to identify and isolate the cause of the high outflow resistance, and thus, adjunctive anatomic study of the graft and its outflow is necessary to identify potentially correctable problems. In most instances, high outflow resistance is due to severe distal disease that cannot be improved. In a few cases, this technique may lead to identification of distal anastomotic problems or the need for extension of a proximal graft to a more distal site for better outflow. In practice, most vascular surgeons have found these techniques somewhat complicated and cumbersome.
Pathogenesis of Graft Thrombosis
Despite meticulous attention to preoperative planning and to technical perfection in the operating room, revascularizations may be unsuccessful. Depending on the type of arterial reconstruction, between 0.3% and 10% fail in the early postoperative period, regardless of attempts by numerous groups to identify and abrogate factors associated with graft failure.1,4,49 Early graft thrombosis has significant prognostic implications, in that a failed reconstruction is associated with poor clinical outcomes, particularly when performed for limb salvage indications.50–52 Several studies have identified a variety of factors potentially contributing to graft failure, including patient demographics, risk factors, comorbid diseases, conduit characteristics, anesthesia type, adjuvant medical therapy, and technical precision.4,53–57 A study from the Washington Hospital Center and Georgetown University Medical Center prospectively collected and analyzed the National Surgical Quality Improvement Program (NSQIP) database from 1995 to 2003 in an attempt to elucidate risk factors predictive of graft failure in patients who underwent infrainguinal arterial bypass. Multivariate logistic regression revealed that younger age (<60 years), African American race, and crural target vessel were associated with graft failure.4 These findings were confirmed in a 2008 NSQIP study by Singh et al4 as well. Furthermore, other modifiable factors predictive of graft failure have been identified and should be incorporated into treatment paradigms. Giswold et al1 documented that dialysis dependency, a known hypercoagulable state, ongoing smoking, and failure to undergo routine graft duplex surveillance were all independently associated with reversed vein graft occlusion.
The role of hypercoagulability as a cause of graft failure has become increasingly recognized in contemporary practice. As the population ages, it has been hypothesized that the number of arterial reconstructions will increase.1,50,58 This, coupled with the introduction and evolution of myriad percutaneous technologies, will lead to an increasing number of interventions that a typical patient will undergo, thereby compounding the impact of hypercoagulability in light of the inherent multiple previous exposures to heparin.59–61
Long-term follow-up has demonstrated that nearly 50% of vascular reconstructions are subject to some degree of restenosis or eventual occlusion,49,62 and thus, has confirmed the efficacy of aggressive graft surveillance and subsequent endovascular or conventional surgical revision in an attempt to identify and prospectively repair deteriorating grafts and prevent graft occlusion52,62–65
When thrombosis does occur, therapeutic alternatives range from expectant supportive care to thrombectomy, thrombolysis, or placement of an entirely new arterial reconstruction. Optimal results in these most difficult circumstances require rapid decisions by the surgeon regarding the etiology of the graft failure and/or thrombosis, selection of either surgical or endovascular repair, timing of re-intervention, and assessment and modification of complex patient risk factors. This discussion summarizes therapeutic guidelines for the most frequently encountered scenarios after thrombosis of an arterial reconstruction.66
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree