Chapter 44
Local Complications
Anastomotic Aneurysms
Kathleen O’Malley Maxfield, Raul J. Guzman
Based on a chapter in the seventh edition by Paul M. Schumacher and Raul J. Guzman
In 1956, Birch et al1 reported the first case of anastomotic aneurysm in a patient after prosthetic aortic graft placement. Since then, anastomotic aneurysms have been recognized as an infrequent, although important, late complication of prosthetic arterial reconstruction. Although true native aneurysmal degeneration may occur at any anastomotic site, most anastomotic aneurysms are considered false aneurysms because they are composed of a fibrous pseudocapsule and not the normal component layers of the arterial wall. Notably, anastomotic aneurysms are associated with potential significant morbidity and mortality, and they present clinical challenges in their detection, evaluation, and management.
Incidence
Overall, anastomotic aneurysms complicate 1.4% to 4% of arterial anastomoses.2 The incidence of anastomotic aneurysms, however, is influenced substantially by anatomic location, surgical technique, time from anastomotic construction, intensity of surveillance, and integrity of the host artery at the original operation.
When reporting incidence, anastomotic aneurysm formation may be described by number of patients, or more accurately, by number of anastomoses at risk. Because anastomotic aneurysms form with the highest incidence at femoral anastomoses, each patient may have two or more sites at risk for aneurysm development, and thus, incidence by anastomotic site appears more informative.3
The interval to presentation of anastomotic aneurysm has changed dramatically.4 Previously an early phenomenon, anastomotic aneurysms in most modern series present at a mean of 6 years following graft implantation.5–10 Associated infection often shortens this interval.11 Ironically, anastomotic aneurysms after reconstruction for aorto-iliac occlusive disease are more likely to have late presentations (15.8 years vs 8.9 years for nonocclusive disease)12 and are more likely to represent degeneration of the anastomosis rather than true aneurysmal change.13
As previously noted, the most common anatomic site of anastomotic aneurysm formation is the femoral artery, where aneurysms occur substantially more often than at aortic or iliac sites. Anastomotic aneurysms have been reported to complicate 0.5% to 23.7% of femoral artery reconstructions.2 In a large retrospective analysis of anastomotic aneurysms after prosthetic reconstructions for aorto-iliac occlusive disease, Van den Akker et al14 reported an incidence per patient of 13.3% and an incidence per anastomotic site of 4.8%, 6.3%, and 13.6% for aortic, iliac, and femoral anastomoses, respectively. Notably, the cumulative freedom from anastomotic aneurysm formation at 15 years was 92.3%, 84.5%, and 76.2% for aortic, iliac, and femoral sites, respectively. This propensity of anastomotic aneurysms to form at the femoral location has been documented by others.3,9
Large retrospective studies of graft-related complications for abdominal aortic aneurysm repair report a cumulative incidence of anastomotic aneurysm of 1.3% to 3.0%.15,16 However, the incidences of aortic and iliac anastomotic aneurysms are probably underestimated because of inadequate surveillance, prolonged time to recognition, and their initially quiescent behavior.17 With extended clinical follow-up, aortic and iliac anastomotic aneurysms may complicate up to 13.3% of anastomoses.18,19 Moreover, studies with routine radiologic surveillance estimate the incidence of anastomotic aneurysms of the aorta and iliac arteries to be approximately 10%, and may reach 27% at 15 years.14,20,21
The documented incidence of anastomotic aneurysms following carotid endarterectomy (with or without patch angioplasty) is approximately 0.3%.22 In numerous series, anastomotic aneurysms represent 13% to 57% of cases of extracranial carotid aneurysms.23 The interval to presentation may occur as early as weeks following carotid intervention. However, the majority of reported cases present from 5 to 12 years after reconstruction. With improved operative technique and the introduction of superior prosthetic materials, carotid anastomotic aneurysms are most commonly associated with surgical site infection, and more specifically, prosthetic infection (see Chapter 103).24
Pathogenesis
An anastomosis between two vascular structures is potentially subject to anastomotic failure, and hence, anastomotic aneurysm formation. However, anastomotic aneurysms occur almost exclusively at anastomoses between prosthetic grafts and native arteries, with only rare occurrences in completely autogenous anastomoses.
When a suture line or anastomosis between two vascular structures is disrupted, an anastomotic aneurysm may form. The egress of blood from the anastomotic defect forms a pulsatile hematoma that, while in continuity with the bloodstream, becomes lined peripherally with laminated thrombus that eventually becomes encapsulated by surrounding host tissue. A fibroblastic process that initiates the formation of a tissue capsule ensues. The capsule, essentially a false aneurysm cavity, is subjected to systemic arterial pressure and may gradually enlarge, occasionally resulting in rupture, local complications of expansion, or distal embolization.
Several etiologic factors have been implicated in the pathogenesis of anastomotic aneurysms, yet the relative importance ascribed to each factor varies substantially by author or institutional experience. Nonetheless, because anastomotic disruption is pivotal in anastomotic aneurysm formation, the pathogenesis may be conceptualized simplistically by considering local and systemic etiologic factors, as listed in Box 44-1. Each factor may induce or contribute to anastomotic failure, and each has assumed prominence during various time periods. Because no robust data exist to corroborate the contribution of some of these factors in the development of anastomotic aneurysms, several factors are accepted on a theoretical basis alone (see Box 44-1).
Local Factors
Arterial Wall Degeneration
The most common etiology of anastomotic aneurysm formation is degeneration of the host arterial wall. Often associated with progression of atherosclerosis, arterial wall degeneration compromises vessel integrity and impairs a critical component of the vascular anastomosis.18,25,26 A consistent operative finding at exploration for anastomotic aneurysm is an intact unit of suture and prosthesis that has separated from an attenuated arterial wall. In a cohort of 49 anastomotic aneurysms, Skourtis et al6 demonstrated the contribution of host arterial degeneration to anastomotic aneurysm development. After anastomotic aneurysm treatment, 28 arterial specimens were examined microscopically and demonstrated reduction or absence of elastic fibers in the media and replacement of smooth muscle cells by acellular fibrous connective tissue. Hyaline degeneration of the media and adventitia was also appreciated. True aneurysmal degeneration of the host artery may be difficult to distinguish from anastomotic false aneurysm development preoperatively.
Suture Line Disruption
Because no durable union occurs between native artery and prosthetic graft, vascular anastomoses depend on the integrity of the suture indefinitely. Suture material, particularly silk, was recognized as a central factor contributing to anastomotic aneurysm formation in early reports. Silk suture gradually dissolves and is eventually resorbed by phagocytosis and other processes, leading to anastomotic failure. This discovery culminated in the abandonment of silk suture for vascular anastomosis. The successors of silk, principally polyethylene and nylon, unfortunately possessed many of the same disadvantages of silk and continued to be implicated in anastomotic aneurysm development, in general because of loss of tensile strength and ultimate suture disruption.27 Monofilament polypropylene (Prolene, Ethicon, Livingston, Scotland) suture was introduced commercially in 1969. It has enjoyed favor among vascular surgeons primarily because of its minimal tissue reactivity, low thrombogenicity, inherent resistance to infection, low coefficient of friction during suturing, and excellent maintenance of tensile strength without biodegradation.28 Despite these advantages, polypropylene suture readily frays and fractures, particularly with instrumentation and indiscriminate handling. Polytetrafluoroethylene (PTFE) suture is preferred by some surgeons because of its favorable suturing characteristics and relatively inert behavior in tissues, although its breaking strength is inferior to that of polypropylene. Nonsuture methods of vascular anastomosis that have recently been explored include rings, staples, clips, tubes, stents, adhesives, and welding. Purported advantages of nonsuture methods include decreased tissue reactivity, diminished vessel trauma, and technical simplicity and efficiency. Only clips are associated with an acceptable complication profile.29,30 Before adoption can become widespread, long-term data are needed, specifically on the incidence of anastomotic aneurysm formation.
Graft Failure
Both early and modern generations of prosthetic grafts have infrequently been incriminated as important factors in anastomotic aneurysm formation. Although both knitted, and less commonly, woven polyester grafts dilate over time, they consistently maintain their structural integrity. Notwithstanding, several investigators have implicated textile graft dilatation and compliance mismatch between the graft and host artery in the pathogenesis of anastomotic aneurysms. Thus, indirectly, prosthetic grafts may contribute to late anastomotic disruption.8,31,32 A potential disadvantage of woven polyester grafts is their tendency to fray with handling. However, incorporation of a greater margin of graft into the anastomosis functionally eliminates this feature as a cause of late anastomotic failure.
Infection and/or Inflammation
Inflammatory states are recognized causes of anastomotic aneurysms. An inflammatory process may occur in response to implantation of prosthetic materials, postsurgical hematoma or lymphocele, or acquired vasculitides. Inflammation caused by acute or indolent graft infection may also lead to anastomotic aneurysm formation (Fig. 44-1). Seabrook et al33 investigated 45 anastomotic aneurysms at the time of surgical intervention for the presence of occult infection despite the absence of clinical signs of anastomotic aneurysm infection. After excision of the false aneurysm and perigraft capsule and subjection to ultrasonic disruption, bacterial isolates were recovered from 60% of specimens. The most common isolate was coagulase-negative staphylococci. The authors surmised that graft contamination, whether early or late, may initiate an inflammatory process with liberation of cytolysins and matrix metalloproteinases that digest adjacent host tissues through fibrinolysis.34 Such an environment may compromise anastomotic tensile strength and predispose to anastomotic aneurysm formation. In a similar study, Downs et al35 analyzed 26 prosthetic grafts by standard histologic and morphologic techniques. Although no clinical infection was evident, the presence of discrete bacteremic colonization was demonstrated in 80% of recovered specimens.
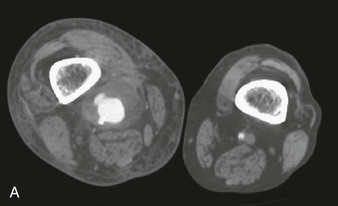
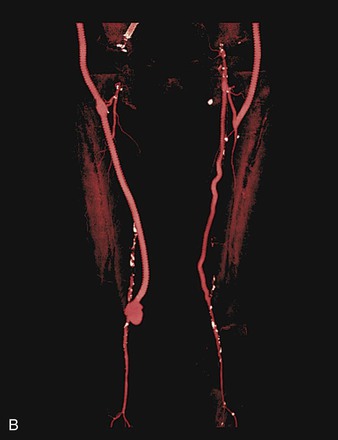
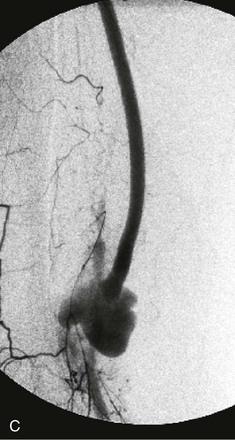
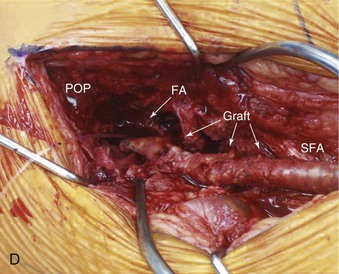
Figure 44-1 Infected anastomotic aneurysm involving the distal femoropopliteal bypass graft. A, Axial computed tomographic images from a preoperative angiogram. B, Reconstructed images from a preoperative computed tomographic angiogram. C, Intraoperative angiogram demonstrating a false aneurysm. D, Degenerated polytetrafluoroethylene graft to the popliteal artery (POP) anastomosis. FA, False aneurysm; SFA, superior femoral artery.
Technical Errors
Refinement in surgical technique has reduced the contribution of technical errors to the development of anastomotic aneurysms. Meticulous attention to suturing, including incorporation of generous portions of the arterial wall, especially in arteries subjected to concurrent endarterectomy, is paramount. Although a putative cause of anastomotic aneurysm, endarterectomy, should be judiciously performed based on its independent merits to ensure graft patency, a surgical technique may require modification. Graft tension during construction of an anastomosis also contributes to late anastomotic disruption and aneurysm formation. Incorrect suture handling, such as grasping the suture with forceps or clamps, may fracture sutures and predispose an anastomosis to late failure. The use of sutures of adequate strength and size also assists in minimizing technical errors.
Mechanical Stress
Myriad mechanical forces operate at an anastomosis and may differentially contribute to anastomotic aneurysm development. Formation of an anastomosis between a prosthetic graft and an artery establishes compliance mismatch. The inherent properties of a prosthetic graft include nondistensibility and a tendency for latent dilatation, both of which impose mechanical stress (shear stress) on an anastomosis. According to Laplace’s law, which states that wall tension increases proportionally with radius and endoluminal pressure, prosthetic dilatation may transmit tension to the suture line and adjacent host artery, resulting in anastomotic disruption. An additional application of this physical principle relates to the diameter of the anastomosis: the greater the diameter, particularly in end-to-side anastomoses, the greater the tension imposed on a suture line.31,32 Extraneous tension on the anastomosis may result in elastic recoil of the graft, leading to separation from the vessel wall.
According to laws of fluid mechanics, the larger the incident angle between the graft and recipient artery, the lower the flow rate through the anastomosis and the greater the associated turbulence and shear stress on the suture line. Hence, constructing an anastomosis with a minimal angle between the host artery and the graft may impart a hemodynamic benefit and prevent late anastomotic complications. Correspondingly, several authors have demonstrated the efficacy of end-to-end, rather than end-to-side, anastomoses in establishing optimal hemodynamic conditions and reduced anastomotic turbulence.26
Systemic Factors
Several systemic factors are thought to contribute to anastomotic aneurysm formation. Smoking, hypertension, and hyperlipidemia, all important risk factors for atherosclerosis, may facilitate anastomotic failure through local effects on arterial wall integrity. Perioperative systemic anticoagulation occasionally complicates the postoperative course through local wound hematoma, a recognized local etiologic factor in anastomotic aneurysm development. Acquired vasculitides are also associated with anastomotic aneurysm development. Behçet disease, an autoimmune vasculitis, is a multisystemic disorder characterized by severe arterial and venous manifestations. Operative intervention in patients with Behçet disease is complicated by anastomotic aneurysm formation in 30% to 50% of these patients because of chronic vasculitis and arterial wall fragility.36 Similar manifestations and complications are observed in Takayasu arteritis36 and in the connective tissue disorder Ehlers-Danlos syndrome type IV.37
Prevention
An understanding of the pathogenesis and risk factors associated with anastomotic aneurysms contributes to their prevention. Deliberate attention to surgical technique is paramount. Anastomoses must be constructed without excessive tension and by incorporating sufficient graft and arterial wall into the suture line, especially if concurrent endarterectomy is performed. Judicious use of adjunctive endarterectomy is advisable. When feasible, an end-to-end anastomosis should be selected unless preservation of critical vascular beds is required (e.g., retrograde iliac perfusion). If an end-to-side anastomosis is performed, a minimum incident angle should be achieved at the anastomotic site.38 In the modern era, the choice of suture and graft material is probably irrelevant and subject to the surgeon’s discretion. Nonetheless, it is recommended that the selected prosthesis have a diameter equal to that of the recipient artery. Strict attention to skin preparation and aseptic operative technique is critical, as is preoperative antibiotic administration. Additional prophylactic strategies include meticulously achieving hemostasis, minimizing lymphatic disruption, avoiding adventitial dissection (i.e., ex-arterectomy), and accurate wound closure.7,9,26 Despite adherence to the aforementioned principles, anastomotic aneurysms are not likely to be preventable, so periodic surveillance should be conducted to prevent related complications. All patients treated with graft material should undergo long-term evaluation, including clinical examination, color Doppler ultrasound, and if necessary, angiographic computed tomography (CT).
Clinical Diagnosis
The diverse clinical manifestations of anastomotic aneurysms are determined largely by anatomic site. Because most anastomotic aneurysms are asymptomatic, they are generally discovered incidentally during routine surveillance or radiologic examinations for unrelated clinical problems.
History and Physical Examination
A careful, systematic physical examination will detect most femoral and other superficially located anastomotic aneurysms. In clinical practice, an anastomotic aneurysm is detected by examination as a palpable, occasionally tender, pulsatile mass. Thus, periodic clinical evaluation with directed physical examination is recommended indefinitely after construction of prosthetic femoral anastomoses. Over a 15-year period, Demarche et al9 retrospectively described their extensive experience with femoral anastomotic aneurysms. Of 142 anastomotic femoral aneurysms, 64% presented as an asymptomatic pulsatile mass, 19% presented with acute limb ischemia, 9% presented as a painful groin mass, and 7% presented with acute hemorrhage. Two patients (1%) presented with distal microemboli and limb edema, respectively. Infection complicated the presentation of 7% of anastomotic aneurysms. The majority of other series reported similar presentations.7,8,39 Thrombosis, embolization, and venous or neurogenic compression are also well-documented complications of femoral anastomotic aneurysms.11
Intra-abdominal anastomotic aneurysms may evade clinical detection until they cause symptoms or enlarge sufficiently that they become palpable. Most aortic and iliac anastomotic aneurysms present as an asymptomatic pulsatile mass or with abdominal or back pain, but they can produce more threatening clinical manifestations such as rupture, infection, distal embolization, erosion with fistulization,40,41 or hemorrhage.5,6 Occasionally, aortic and iliac anastomotic aneurysms are discovered incidentally during radiologic examinations for unrelated clinical problems. Many large intra-abdominal anastomotic aneurysms are palpable on physical examination, particularly in nonobese patients. Small or intracavitary anastomotic aneurysms, however, are often not appreciated by physical examination and require focused radiologic studies for diagnosis.
Many carotid anastomotic aneurysms display progressive asymptomatic enlargement. However, local compression, distal embolization, and even rupture are notable manifestations. Neurologic symptoms are particularly common and are present in 14% to 74% of patients in various series. Rupture is fortunately infrequent, but may occur in approximately 3% of cases.23
Imaging
During surveillance or when an anastomotic aneurysm is suspected but cannot be confirmed by examination, ultrasonography, computed tomographic arteriography (CTA), and magnetic resonance arteriography (MRA) are reliable diagnostic modalities. The quality and resolution of modern CTA and MRA often obviate the need for conventional catheter-based arteriography (Fig. 44-2). Moreover, some consider conventional arteriography unreliable because of its inability to identify anastomotic aneurysms obscured by thrombus and because noninvasive arteriographic methods more accurately assess aneurysm size and guide recommendations for intervention.42,43 To determine the validity of several current vascular imaging modalities in diagnosing aortic anastomotic aneurysms, Bastounis et al44 performed a cross-sectional study comparing B-mode ultrasonography, CT, magnetic resonance imaging (MRI), and digital subtraction angiography (DSA). Defining color-coded Doppler imaging as the “gold standard,” they determined that all modalities had sensitivities of 100%, but only MRI, DSA, and enhanced CT possessed sufficient specificity, and only MRI possessed sufficient positive predictive value for diagnostic utility. Although interesting, this study’s applicability is limited by the small number of anastomotic aneurysms encountered and by modern technologic improvements in CT and MRI scanners.
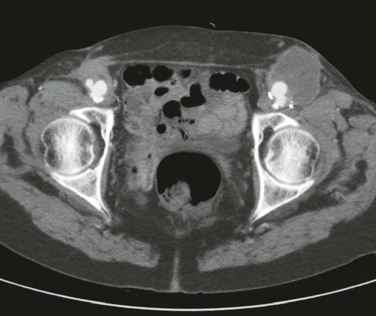
Figure 44-2 Axial computed tomographic image of bilateral femoral anastomotic aneurysm after aortobifemoral bypass.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


