Chapter 48
Local Complications
Nerve Injury
Sara Clark, Robert Atnip
Based on a chapter in the seventh edition by Robert P. Garvin and Robert G. Atnip
In vascular surgery patients, nerve damage can arise from several different mechanisms: acute or chronic ischemic damage to nerves (ischemic neuropathy), trauma (e.g., from surgical trauma or compression from a hematoma), and associated co-morbid disease (e.g., diabetes or uremia).
This chapter describes (1) ischemic neuropathy, an underrecognized form of peripheral nerve damage that must be distinguished from ischemic pain; (2) nerve trauma, which can be prevented by knowledge of the relevant anatomy; and (3) other neuropathies encountered in vascular patients because of their associated diseases.
Structure of a Peripheral Nerve
The peripheral nerves are composed of nerve fibers, Schwann cells, collagen, small vessels, and endoneurial fluid. Large nerves derive their nutrient and oxygen supply from the vasa nervorum, whereas smaller nerves are able to rely on diffusion through the interstitial space to supply their metabolic demands. Although the function of the nerve to conduct an action potential through tightly regulated ionic gradients along the axon is dependent on energy in the form of adenosine triphosphate, the oxygen requirement of a mammalian nerve is small. Even when the requirement is increased by activity, it is less than that of other tissues.1 Hence, peripheral nerves tend to be resistant to ischemia. The large nerves of the extremities contain multiple redundant collateral networks of vasa nervorum along the length of the axons that are supplied by sequential branches of adjacent arteries. The inner portions of the nerves contain an extensive pattern of epineural vessels.2,3 The result of this rich collateralization is that interruption of the blood supply to a segmental portion of the nerve does not usually result in ischemic nerve injury.4,5
Causes of Nerve Damage
Ischemic Nerve Injury
The true incidence of ischemic neuropathy in a patient with peripheral arterial disease (PAD) is difficult to determine because the symptoms of ischemic nerve injury may mimic the symptoms of arterial insufficiency, such as pain at rest or claudication, or the symptoms of diabetic neuropathy, such as decreased sensation. Older studies have estimated the incidence of clinically detectable ischemic neuropathy to be as high as 88% in patients with PAD.6 This high incidence was confirmed by Weinberg et al,7 who reported an 84% incidence of pain and sensory disturbances in a cohort of patients with PAD, with the majority of patients having sensory deficits. These percentages are even more staggering when electrophysiologic nerve conduction studies are used to document occult nerve damage in patients with PAD; several authors have reported a nearly universal presence of detectable nerve dysfunction.8–10
The mechanism of ischemic injury in patients with PAD has not been fully elucidated. Both acute and chronic ischemic conditions have the potential to cause nerve injury, but experimental models of chronic ischemia are lacking.
Acute Ischemia
Animal models of acute limb ischemia have demonstrated the ability of injured mammalian nerve tissue to recover after short periods of ischemia subsequent to occlusion of large inflow arteries11 and smaller vasa nervorum.12 There is, however, a certain time threshold in animal models, between 6 and 10 hours, beyond which the neurologic damage is irreversible.13,14 During the initial period of acute ischemia it is believed that peripheral nerve tissue is able to resist ischemic injury by its inherently low metabolic demand, by recruiting oxygen and nutrient supply from the surrounding interstitial fluid, and by the rich collateral circulation within the nerve bundle itself. This period of resistance is eventually overcome by persistent hypoxemia and hypercapnia, which lead to hyperkalemia and acidosis. Hyperkalemia alone in the local milieu of the ischemic nerve may contribute to irreversible depolarization of the axon cell membrane and results in acute nerve dysfunction.15,16 Exactly how much ischemia the human peripheral nerve is able to tolerate without irreversible injury is still unknown.
Chronic Ischemia
In the absence of experimental models of chronic limb ischemia, several investigators have attempted to describe the histologic findings of peripheral nerve specimens obtained from patients with chronic PAD. Evidence of segmental demyelination and remyelination, as well as axonal degeneration and regeneration, was demonstrated histologically in patients with chronic PAD several decades ago by Eames and Lange.6 These findings have been confirmed in more recent studies by other investigators.17–19
The mechanism by which chronic limb ischemia produces changes in the peripheral nerve is still not known; however, Nukada et al19 were able to demonstrate similar morphologic changes in peripheral nerves affected by both acute and chronic ischemia, thus suggesting that the lack of blood flow and oxygen delivery has the same ultimate effect whether it happens acutely or slowly over time. The severity of the chronic ischemia so far has not been shown to be predictive of the degree of histologic damage found in peripheral nerves. When Farinon et al17 published their observations of segmental demyelination and axonal degeneration in nerve biopsy specimens from patients with chronic PAD, there was no correlation with the severity of the accompanying PAD. Furthermore, the injury observed in chronically ischemic limbs may not be a result of slow progression of the ischemic process but rather the accumulation of multiple repeated episodes of acute ischemia in the setting of chronic PAD, as suggested by Lacroix et al.20 This idea fits with a more recent study that demonstrated that ischemic preconditioning, which tends to be beneficial to skeletal muscle tissue, is harmful to peripheral nerve tissue.21
Traumatic Nerve Injury
The anatomic proximity of several peripheral nerves to the major arteries and veins of the extremities places these nerves at risk for iatrogenic injury during vascular interventions. Without a commanding knowledge of the anatomic relationships of structures within the major neurovascular bundles, vascular surgeons may successfully restore flow to an extremity yet leave significant neurologic deficits because of surgical trauma. Fortunately, most peripheral nerve injuries that occur during operative exposure result in transient nerve dysfunction (neurapraxia) that recovers with time.
Traumatic injury can occur via several mechanisms. Direct division or ligation of a nerve renders the nerve immediately and permanently damaged. Although reconstruction of divided nerves is possible with acceptable rates of functional recovery,22,23 the outcome is hardly optimal. Other mechanisms include stretch injury from improper retractor placement and thermal injury from electrocautery devices. Compressive injury can result from hematoma formation or from improper patient positioning on the operating table. Nerves can also be injured by clamps or entrapped by sutures. The ability to avoid nerve injury during operative procedures is based on a high level of technical precision with constant vigilance for anatomic relationships. Except in situations in which the nerve is completely severed, the capacity of peripheral nerves to regenerate is such that functional outcome in most instances is excellent.
Other Causes of Nerve Injury
A proportion of patients evaluated by a vascular specialist will have peripheral neuropathy that is not due to ischemia or trauma (Box 48-1). It is important to include these other causes in the differential diagnosis so that appropriate neurologic consultation can be obtained for patients deemed not to have ischemic or traumatic neuropathy.
Diabetic neuropathy is the most commonly encountered alternative cause of peripheral neuropathy seen by vascular specialists. Up to 34% of diabetics will develop some form of neuropathy during their lifetime.29 Risk factors are increasing age, increasing length of time being diabetic, poor overall glycemic control, poor lipid metabolism, inadequate blood pressure control, obesity, and metabolic syndrome.29–31 The etiology of the nerve damage is multifactorial and not completely understood, but the metabolic derangements associated with chronically elevated blood glucose levels and abnormal insulin homeostasis are thought to be the major determinants.32 Vascular disease in the microcirculation is also believed to play a role in nerve damage, thus making the difference between diabetic neuropathy and ischemic neuropathy less distinct.33
Other causes of peripheral neuropathy include alcoholism, uremia, drug intoxication, medication side effects, vasculitis, autoimmune disorders, infectious causes, and inflammatory causes. Most often these neuropathies are symmetric.
Diagnostic Evaluation
Diagnostic evaluation of any vascular surgical patient begins with a thorough history and physical examination.
History
Particular attention should be paid to the type of pain that the patient describes.
The quality of the pain of intermittent claudication can be highly variable, but is typically described as a cramping or tightening sensation, usually in the calf or more proximal muscles, and consistently occurring with a predictable amount of exertion and relieved by briefly resting the muscles.
Ischemic pain at rest, as the name implies, occurs at rest, is most often unilateral, involves the foot and perhaps the calf, is usually worse at night, and is generally relieved by placing the extremity in a dependent position to allow gravity to assist in blood flow. If patients are ambulatory, they also have claudication.
Neuropathic pain is usually constant, but may be worse at rest. It typically has no particular aggravating or alleviating factors. It is often described as a shooting or burning pain associated with the sensation of numbness and tingling, and is most severe distally in the toes or foot. These symptoms are unilateral in ischemic neuropathy but are bilateral and fairly symmetric in metabolic neuropathies.
After revascularization of a severely ischemic limb, it is not unusual for patients to complain of persistent pain that is due now to neuropathy rather than ischemia. The neuropathic pain is burning and paresthetic, is frequently worse with rest and at night, and is unaffected or relieved by walking, in marked contrast to the pain of claudication. The patient perceives the foot to be cold, although it is, in fact, warm. The patient may remark on loss of mobility of the toes.
Physical Examination and Noninvasive Testing
Vascular Testing
Physical examination should include a detailed vascular examination supplemented by noninvasive vascular testing to document the degree of arterial ischemia.
If ankle pressure is greater than 50 to 60 mm Hg or toe pressure is greater than 30 mm Hg, ischemic pain at rest is unlikely, and a diagnosis of ischemic neuropathy should be suspected. Flat or monophasic Doppler waveforms confirm that the arterial disease is severe. If clinical examination and noninvasive assessment confirm that the peripheral circulation is adequate, the pain is probably not due to ischemia. If perfusion is inadequate, the pain may be due to ischemia, neuropathy, or both, and treatment should be directed first toward improvement of limb blood flow.
If no ischemia is present, a neurologic evaluation should be performed. If ischemia is present, efforts should be made to correct the ischemia in parallel with any indicated neurologic testing.
Neurologic Testing
Examination does not reveal signs of significant ischemia. The small muscles of the affected foot are wasted in comparison to those on the normal side, and they are weak. There may be slight ankle weakness, and the ankle reflex may be depressed compared with the normal side. There is unilateral sensory loss in a “stocking” distribution, particularly vibration sense. Unlike the findings in neuropathies caused by diabetes, uremia, drug intoxication, or alcoholism, the findings in ischemic neuropathy are asymmetric, with sensory and motor findings exclusively or prominently in the limb that is affected by severe ischemia.
The peripheral neuropathy associated with diabetes is characterized by bilateral sensory loss, numbness and tingling, and pain in the hands, feet, fingers, and toes. Distal muscle weakness and muscle wasting are common, and ankle jerks are absent. The pattern of distribution is symmetric over the involved extremities, in contrast to the unilateral pattern of ischemic neuropathy.
Evaluation of individual nerves that can be injured by trauma is discussed later under “Specific Nerve Injuries.”
Electromyography and Nerve Conduction Studies
Careful electrophysiologic studies can establish the diagnosis of ischemic neuropathy and other neuropathies and define their severity. Neurologic testing consists of motor nerve conduction studies, sensory nerve conduction studies, and needle electrode examination. With ischemic neuropathy, patients typically have a unilateral axonal neuropathy involving the distal nerves.
Motor nerve conduction studies show a decrease in or absence of the compound muscle action potential amplitude from the extensor digitorum brevis muscle when the peroneal nerve is stimulated and from the flexor hallucis brevis muscle when the posterior tibial nerve is stimulated in the affected foot.34 Frequently, the distal posterior tibial nerve is involved more than the distal peroneal nerve. The abnormality is always most severe in the distal nerves. Distal latency, if one can be recorded, and the velocity of conduction in the calf portion of the peroneal and posterior tibial nerves are relatively well preserved. These findings are in sharp contrast to those in diabetic and uremic neuropathy, in which distal latencies and conduction velocities tend to be symmetrically reduced below normal velocities at an early stage in both lower limbs.
Sensory nerve conduction studies show decreased or absent sensory potential amplitudes from the sural, superficial peroneal, and plantar nerves, whereas sensory conduction velocity, when recordable, is normal.34,35
Needle electrode examination reveals the changes of muscle denervation in the small muscles of the affected foot, particularly in the sole of the foot, with fibrillation potentials at rest and large motor units of long duration in much reduced numbers. Lesser denervation changes are seen in the muscles of the calf if the ischemia has been severe.36
General Principles of Treatment
Acute Ischemic Neuropathy
If a patient has acute arterial ischemia, revascularization will often improve the motor and sensory symptoms of ischemic neuropathy either immediately or over the first few weeks. If symptoms persist longer than a few weeks, and the revascularization is deemed adequate, a neurologic cause of the pain should be suspected. The pain should be treated, and a neurologic consultation obtained. Treating the pain of ischemic neuropathy is often difficult because the typical narcotic regimens used for postsurgical pain may not be effective for the treatment of ischemic pain and because other drugs have variable responses in patients with peripheral neuropathy.
Basic science research is currently focusing on the role of neuroprotection for patients with acute limb ischemia. Wang et al34 demonstrated less sciatic nerve damage in tumor necrosis factor-α knockout mice than in wild-type mice in a model of ischemia-reperfusion injury, and Ramaglia et al35 demonstrated a protective effect of soluble complement receptor-1, a potent inhibitor of all complement pathways, in a crush injury model of the rat sciatic nerve. These studies evaluated modulation of the immune system and the inflammatory response to acute ischemic nerve injury. Whether they will prove to be effective protection for patients with acute limb ischemia or nerve injury (or both) remains to be seen.
Pain Control
Ideally, the pain from ischemic neuropathy should be managed by a neurologist or other specialist familiar with treating this condition. Anti-inflammatory drugs and opiate medications are generally ineffective. Antidepressants, particularly tricyclics (amitriptyline) and some antiepileptics (gabapentin and pregabalin), have been empirically found to be beneficial but do not provide reliable relief for all patients.
Trauma
A patient with partial loss of nerve function after surgery can generally expect to recover, although the recovery period may be lengthy. In a patient with severe loss of motor and sensory function, surgical re-exploration should be considered. If a major nerve or branch is found to be divided, repair is indicated. If a nerve is acutely compressed because of a hematoma, surgical decompression should be performed promptly and will often result in recovery of function.
In cases in which symptoms persist without signs of healing and nerve conduction studies indicate loss of nerve function, surgical exploration with neurolysis and nerve grafting offers some hope of recovery of function, with encouraging results being reported in contemporary series.23–28
Management of trauma to individual nerves is discussed in the next section.
Specific Nerve Injuries
Brachial Plexus and Upper Extremity
Anatomy
The brachial plexus gives rise to the five main terminal nerves of the upper extremity: the axillary nerve (C5, C6), the musculocutaneous nerve (C5, C6), the median nerve (C5-T1), the radial nerve (C5-C8), and the ulnar nerve (C8, T1).
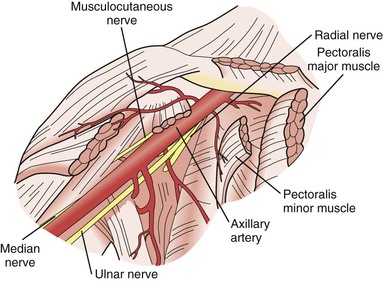
The superior, middle, and inferior trunks of the brachial plexus are intimately associated with the axillary artery as they make the turn along the shoulder joint and become the lateral, posterior, and medial cords. Great care must be taken during operative exposure of the axillary artery to not injure the cords of the brachial plexus because they are located on three of the four sides of the artery at that point (Fig. 48-1).
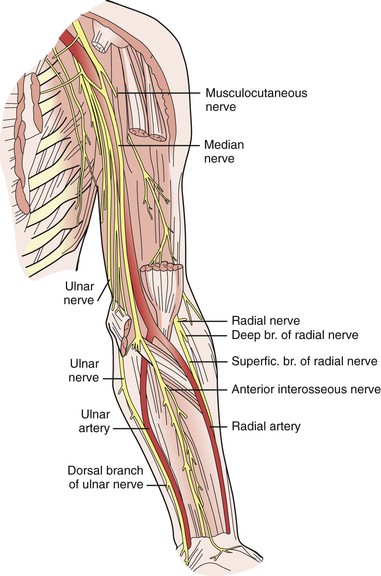
The musculocutaneous and axillary nerves terminate in the upper part of the arm and are not routinely encountered during vascular exposure there. Likewise, the radial nerve is rarely encountered in its deep location posterior to the humerus as it courses toward the extensor compartment of the forearm. However, in the upper part of the arm, the median and ulnar nerves are intimately associated with the brachial artery and basilic vein (Fig. 48-2). The proximity of the median nerve as it wraps around the brachial artery in a lateral-to-medial direction must be appreciated during exposure of this artery to avoid iatrogenic injury; likewise, the position of the ulnar nerve deep to the basilic vein must be remembered when dissecting this vein free from surrounding tissue.
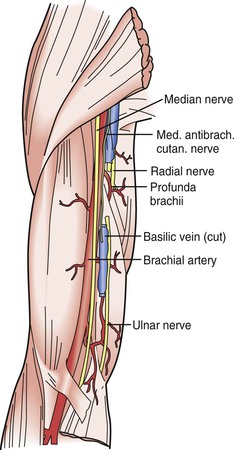
Figure 48-2 Relationship of the brachial artery and basilic vein to the median and ulnar nerves in the neurovascular bundle that runs deep to the medial brachial fascia.
In the forearm, the median nerve is not routinely encountered, whereas the ulnar nerve, which runs adjacent to the ulnar artery into the hand, can be injured when the distal ulnar artery is dissected free. The location of the ulnar nerve at the elbow also makes it vulnerable to compression injury from improper positioning of the patient’s arm during surgery (Fig. 48-3).
Etiology
The most common causes of injury to nerves in the upper extremities include direct surgical injury, compression, and traction. The more common procedures associated with injury include hemodialysis access, arterial puncture for diagnosis or monitoring, radial artery harvesting, and venipuncture.
Surgical Dissection.
During thoracic outlet surgery, the brachial plexus can be injured by direct trauma or traction (see Chapter 126). When exposing the axillary artery, such as for axillofemoral bypass, injury can occur to the cords of the brachial plexus and result in a neurologic deficit that affects more than one peripheral nerve.
In the upper part of the arm, the median nerve with its close proximity to the brachial artery and the ulnar nerve with its close proximity to the basilic vein can be injured by dissection. The median and ulnar nerves in the upper part of the arm and forearm can also be injured during surgical dissection of the major arteries.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree