10 Lipid Lowering in Coronary Artery Disease
 Epidemiology
Epidemiology
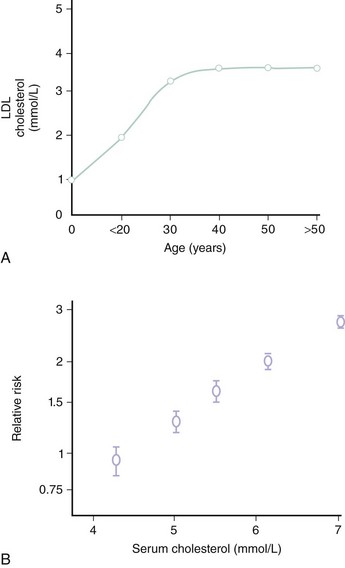
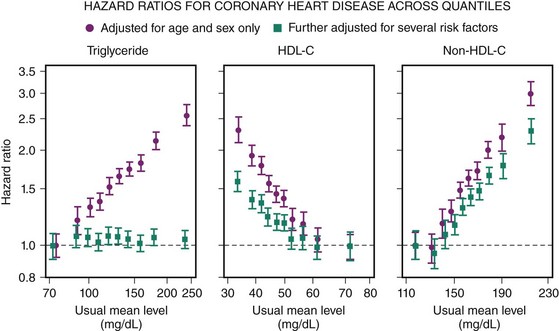
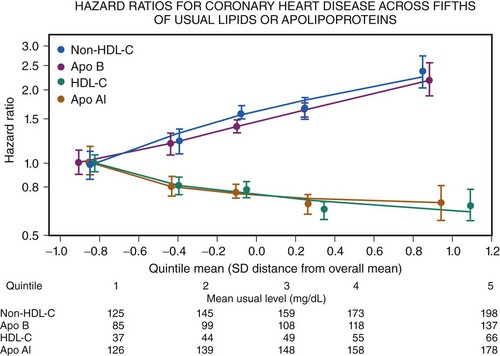
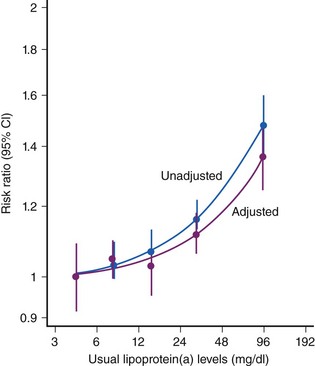
CAD is the largest cause of premature death in the Western world (Fig. 10-1). A person is born with an LDL cholesterol (LDL-C) level of 0.8 mmol/L, which increases throughout life (Fig. 10-2, A). Several epidemiologic studies have demonstrated a relationship between elevated total cholesterol and LDL-C and an increased risk of death or nonfatal myocardial infarction (MI).1–4 The relationship between cholesterol and risk of CAD is linear, with no apparent threshold below which risk declines (see Fig. 10-2, B), which suggests that interventions that reduce cholesterol the most are also likely to have the greatest impact on CAD risk reduction.5 The central role of cholesterol in the pathophysiology of CAD has pushed lipid-lowering therapy to the forefront of medical management of this condition. More recently, the largest observational study to date involving over 1 million person-years worth of observation and more that 10,000 cases of fatal or nonfatal MI has shed further light into the relevance of simultaneous assessment of triglycerides (TGs), HDL-cholesterol (HDL-C) and non–HDL-cholesterol (non-HDL-C), which consists of very-low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), and LDL-C.6 Specifically, this shows that after adjustment for non–HDL-C and HDL-C, there is no association between TG and risk of CAD (Fig. 10-3). In contrast, the inverse association between HDL-C and risk persists with some attenuation above 70 mg/dL. One practical implication of this observation is that among individuals with an HDL-C >70 mg/dL use of the TC/HDL-C ratio underestimates cardiovascular (CV) risk in risk calculators. As with LDL-C, a positive relationship is observed between non–HDL-C and risk of CAD with little attenuation. No difference has been observed between fasting and nonfasting levels and risk, suggesting that lipid measurements can be simplified by using non–HDL-C (the difference between TC and HDL-C) and HDL-C levels. Data suggest that TGs are unlikely to be causative factors but markers of risk and that the risk is conferred by concomitant low levels of HDL-C and high levels of non–HDL-C. These latter two factors, rather than TGs, should be the targets of treatment in people with elevated TG levels, providing independent support for the NCEP goals. The LDL-C level incompletely measures atherogenic lipoproteins; measurement of the concentration of apolipoprotein B (apoB), which is a direct measurement of the concentration of proatherogenic particles (e.g., LDL-C, VLDL, IDL), and measurement of apoAI, the major protein in HDL particles, provide alternative approaches to risk prediction. The debate regarding the choice of the best lipid parameter has further intensified with apparently conflicting evidence between prospective studies.7–9 In approximately 100,000 individuals with about half a million person-years worth of follow-up, it is now clear that overall apoB (the major lipoprotein) in atherogenic particles is equivalent to non–HDL-C and that apoAI, the major protein in HDL particles, is equivalent to HDL-C (Fig. 10-4); this suggests that the choice of assays should be informed by availability and cost. Lipoprotein (a) (Lp[a]) consists of one apoB particle covalently bonded to a protein apolipoprotein via a disulfide bond. It is produced by the liver and is principally under genetic control, which makes it the most reproducible biomarker known. Elevated Lp(a) levels can potentially increase the risk of cardiovascular disease (CVD) (1) via a prothrombotic/anti-fibrinolytic effect as apolipoprotein (a) possesses structural homology with plasminogen and plasmin, and (2) via accelerated atherogenesis as a result of intimal deposition of Lp(a) cholesterol. Observational data from 126,634 individuals suggest a curvilinear association between levels and risk of CAD and stroke even after adjustment for conventional risk factors and other lipid fractions (Fig. 10-5).10 This association, however, is modest and per 1 SD (standard deviation) difference, that is, about a 60% higher level Lp(a) increases risk by about 13%. It is likely that this factor is causally related and specific for CVD as there is no association with nonvascular deaths (a prior requisite of the Bradford-Hill Criteria for causality); recent mendellian randomization data have provided consistent data for an association of Lp(a) genotype with Lp(a) levels and, in turn, between genotypes and CAD risk.11
 National Cholesterol Education Program Recommendations
National Cholesterol Education Program Recommendations
To help reduce the prevalence of elevated blood cholesterol levels in adult Americans, the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) launched the National Cholesterol Education Program (NCEP) in 1985.12 For the first time, there was a consensus among leading experts in the field on the measurement, detection, and treatment of patients with hypercholesterolemia. The report established criteria that defined candidates with high blood cholesterol levels who should receive medical intervention and provided guidelines on how to detect, set goals, treat, and monitor these patients over time. The NCEP-1 treatment guidelines recommended that all adults older than 20 years have a blood cholesterol measurement at least once every 5 years. Patients with levels greater than 200 mg/dL (5.2 mmol/L), confirmed by a second blood cholesterol measurement, were advised to adopt a Step 1 fat-controlled diet. Patients with cholesterol exceeding 240 mg/dL (6.2 mmol/L) were candidates for intensive treatment with a Step 2 diet and sometimes with drugs, as were those with cholesterol levels in the range of 5.2 to 6.2 mmol/L (200 to 240 mg/dL), who were at especially high risk because they already had CAD or two other risk factors. It was also recommended that drugs for lowering blood cholesterol should be used only when the indication has been confirmed by measuring LDL-C and as a supplement to the dietary treatment. These guidelines were the first steps toward the management of lipids that have become commonplace today.
 Early Nonstatin Lipid-Lowering Trials
Early Nonstatin Lipid-Lowering Trials
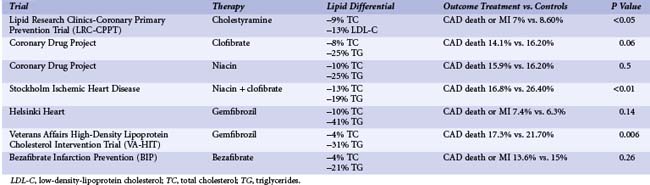
In the Lipid Research Clinics–Coronary Primary Prevention Trial (LRC-CPPT), cholestyramine therapy resulted in a 13% reduction in LDL-C and a significant 19% reduction in fatal and nonfatal MI at 7 years (Table 10-1).13 During the first 2 years of the trial, higher event rates occurred in the cholestyramine group compared with the placebo group. The Coronary Drug Project evaluated the effects of estrogen, dextrothyroxine, clofibrate, and niacin on recurrent disease in men. Clofibrate resulted in an 8% reduction in total cholesterol and a 25% reduction in triglycerides but no significant reduction on the combined endpoint of cardiac death and nonfatal MI at 5 years.14 Subjects who were assigned to niacin treatment achieved a 10% reduction in total cholesterol and a 25% reduction in triglyceride levels. At 5 years, a dose of niacin (3 g/day) was associated with a significant reduction in CAD death or MI (25.6% vs. 30.1%, P < 0.005).15 Benefit was not evident after the second year of therapy. The Stockholm Ischaemic Heart Disease Prevention study evaluated the combination of niacin (3 g/day) and clofibrate (2 g/day).16 Total mortality and, notably, CAD mortality were significantly reduced in the lipid-lowering therapy group (16.8% and 21.9% vs. the control group rates of 26.4% and 29.7%, P < 0.05 and P < 0.01, respectively). A significant reduction in nonfatal MI was reported at 44 months (6.8% vs. 13.6%, P < 0.01). No significant benefit from gemfibrozil therapy was observed in the secondary prevention arm of the Helsinki Heart Study.17 However, gemfibrozil therapy did reduce the rates of MI and CAD death in men in the secondary prevention Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT).18
 Early Secondary Prevention Statin Trials
Early Secondary Prevention Statin Trials
During a decade of clinical research, successive trials using statins have demonstrated the benefit of lowering serum cholesterol in a wide range of clinical conditions compared with diet alone. The first of these trials was the Scandinavian Simvastatin Survival Study (4S) trial, which randomized 4444 patients with angina pectoris or previous MI and serum cholesterol levels of 215 to 312 mg/dL (5.5 to 8.0 mmol/L) to a lipid-lowering diet or to treatment with simvastatin (average dose of 20 mg/day).19 Over 5 years, simvastatin produced mean reductions in total cholesterol and LDL-C levels of 25% and 35%, respectively. Statin therapy was associated with an absolute 4% reduction in mortality and a relative risk reduction in all-cause mortality of 30% (P = 0.0003). Significant reductions were also observed for CAD death (42%), major CAD event (34%), and the need for revascularization (37%). This was the first trial in the modern era that provided definitive proof that lipid-lowering therapy was safe and reduced the risk of cardiac death or nonfatal MI. The Cholesterol and Recurrent Events (CARE) trial quickly followed 4S with consistent findings, demonstrating a reduction in major coronary events with pravastatin (40 mg) versus placebo, as well as reductions in the rates of revascularization and stroke in patients with normal cholesterol levels.20 The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) trial was the largest of the three early secondary prevention trials (N = 9014 patients), and it confirmed the mortality findings of 4S in a population with an overall lower total cholesterol level.21 LIPID demonstrated that among patients with a history of MI or hospitalization for unstable angina and initial plasma total cholesterol levels of 155 to 271 mg/dL (3.97 to 6.95 mmol/L), pravastatin (40 mg/day) reduced CAD death by 1.9%, resulting in a 24% relative risk reduction (P < 0.001). Similarly, overall mortality was reduced (22%), as were rates for recurrent MI (29%), stroke (19%), and coronary revascularization (20%). These three trials demonstrated the cumulative benefit of statins across a range of baseline cholesterol values.
 Heart Protection Study and Cholesterol Treatment Trial Meta-Analysis
Heart Protection Study and Cholesterol Treatment Trial Meta-Analysis
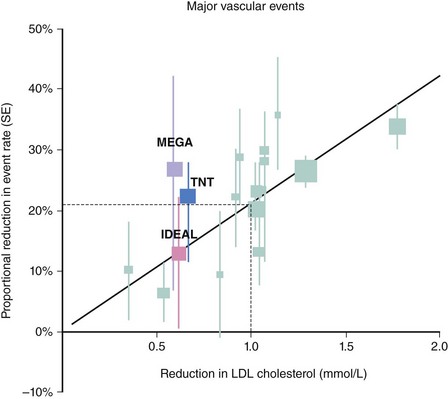
The large Heart Protection Study (HPS) demonstrated that the magnitude of benefit from statin therapy (40 mg of simvastatin) was similar at each level of baseline LDL-C, including subjects with an LDL-C level below 100 mg/dL (2.56 mmol/L).22 HPS studied approximately 20,000 patients who were able to tolerate simvastatin (after a run-in phase) over 5 years and assessed the on-treatment effect (rather than the intention-to-treat effect) of a standard dose of a statin rather than a specific cholesterol or LDL-C target. HPS, however, included a range of patients with CAD; those with prior vascular disease, such as peripheral vascular disease or stroke; and subjects without prior clinical manifestations of vascular disease who were considered at high risk due to diabetes.23 The implications of this trial for clinical practice were that physicians did not need to treat to specific targets because subjects with vascular disease all derived similar proportional reductions in risk with simvastatin (40 mg). Preliminary evidence indicated that some overall benefit existed for statin therapy in different circumstances, such as different ages, genders, and different levels of established risk factors, but additional data involving several thousand more patients were needed to provide large-scale evidence of benefit in individual subgroups. The Cholesterol Treatment Trial (CTT) collaborators set out to undertake a prospective meta-analysis of mortality and morbidity from all relevant large-scale, randomized trials of statin therapy.24 Data on 90,056 individuals were combined. During a mean follow-up of 5 years, there was a significant 12% reduction in all-cause mortality per 38.6-mg/dL (1-mmol/L) reduction in LDL-C, a 19% reduction in coronary mortality, a 24% reduction in the need for revascularization, and a 17% reduction in stroke. Overall, a 38.6-mg/dL (1-mmol/L) reduction in LDL was associated with a 21 to 23% reduction in any major vascular event (Fig. 10-6). Importantly, a similar proportional benefit was observed in different age groups, across genders, at different levels of baseline lipids (including triglycerides and high-density lipoprotein cholesterol [HDL-C]), and equally among those with prior CAD and cardiovascular risk factors and in those without. The CTT meta-analysis collected data on 5103 new cases of cancer, with no evidence that statins increased the overall incidence of any form of cancer (hazard ratio [HR], 1.0; P = 0.9).
 Intensive Statin Therapy for Acute Coronary Syndrome
Intensive Statin Therapy for Acute Coronary Syndrome
The early trials looking at treatment of acute coronary syndrome (ACS) (i.e., 4S, CARE, and LIPID) excluded patients within the first 4–6–month period after ACS. The Myocardial Ischemia Reduction with Acute Cholesterol Lowering (MIRACL) trial provided the first evidence that statin therapy initiated early after ACS reduced adverse clinical events by 16 weeks.25 However, the higher-than-usual dose of statin (80 mg of atorvastatin) and lack of an active comparator coupled with an absence of long-term safety data limited the widespread applicability of its findings.
PROVE IT-TIMI 22 Trial
The Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis In Myocardial Infarction 22 (PROVE IT-TIMI 22) was the first large-scale study of statin therapy comparing two active comparators. In the PROVE IT-TIMI 22 trial, patients who had been hospitalized for ACS within the preceding 10 days (N = 4162) were randomized to 40 mg of pravastatin (i.e., standard therapy) or 80 mg of atorvastatin daily (i.e., intensive therapy).26 The primary endpoint was a composite of death from any cause, MI, documented unstable angina requiring rehospitalization, revascularization (performed at least 30 days after randomization), and stroke.
A to Z Trial
The Aggrastat to Zocor study (A to Z trial) compared an early initiation of an intensive statin regimen with delayed initiation of a less-intensive regimen in patients with ACS.27 Patients with ACS (N = 4497) received 40 mg of simvastatin for 1 month, followed by 80 mg thereafter, which was compared with placebo for 4 months followed by 20 mg of simvastatin. The primary endpoint was a composite of cardiovascular death, nonfatal MI, re-admission for ACS, and stroke. The median LDL-C level on placebo was 122 mg/dL (3.16 mmol/L) at 1 month and 77 mg/dL (1.99 mmol/L) at 8 months with 20 mg of simvastatin. The median LDL-C concentration achieved at 1 month with 40 mg of simvastatin was 68 mg/dL (1.76 mmol/L) and 63 mg/dL (1.63 mmol/L) at 8 months with 80 mg of simvastatin. Overall, 16.7% in the placebo plus simvastatin group experienced the primary endpoint, compared with 14.4% in the simvastatin-only group (40 mg/80 mg), reflecting an HR of 0.89 (P = 0.14). Myopathy (i.e., creatine kinase >10 times the upper limits of normal with muscle symptoms) occurred in 9 patients (0.4%) receiving 80 mg of simvastatin, in no patients receiving lower doses of simvastatin, and in 1 patient receiving placebo (P = 0.02). The PROVE IT-TIMI 22 and A to Z trials compared similar intensive and moderate statin therapy after ACS, with apparently disparate results. An analysis comparing and contrasting the two trials observed differences between the trials in baseline demographic characteristics, geographic locations, and use of percutaneous coronary intervention (PCI).28 The LDL-C level difference was greater early in the A to Z trial than in PROVE IT (≤4 months), but the difference was less late in the trials. Significant C-reactive protein (CRP) reduction also occurred earlier in PROVE IT. With common endpoints, an early favorable separation of event curves was seen in PROVE IT but not in the A to Z trial. Clinical endpoint rates and reductions were similar in both trials after 4 months. Factors that may explain this disparity include the statin regimen in the early phase, leading to differences in the magnitude of LDL-C lowering and CRP levels and differences in the early use of PCI. In summary, the results of these trials support a strategy of early, intensive statin therapy coupled with revascularization when appropriate in patients after ACS.28
 Intensive Statin Therapy in Stable Coronary Artery Disease
Intensive Statin Therapy in Stable Coronary Artery Disease
Despite the landmark results from PROVE IT, which led to NCEP recommending an optional LDL-C goal of less than 70 mg/dL in high-risk patients, several questions arose, such as whether intensive statin therapy was safe over a longer period and whether intensive statin therapy was as beneficial in subjects with stable CAD as in ACS patients.29 The Treating to New Targets (TNT) trial and the Incremental Decrease in Endpoints through Aggressive Lipid Lowering (IDEAL) trial addressed these issues and provided approximately 50,000 patient-years of data on the safety and efficacy of intensive statin therapy.30,31
IDEAL Trial
The Incremental Decrease in Endpoints through Aggressive Lipid Lowering (IDEAL) trial was a randomized, open-label trial that compared a strategy of achieving an approximately 35% reduction in LDL-C using the 20-mg/40-mg dose of simvastatin versus a strategy of achieving a 55% reduction in LDL-C using 80 mg of atorvastatin in patients with a history of MI. Patients were recruited months to years after the index MI, making IDEAL comparable in design to the 4S and CARE trials.19,20 Approximately 70% of patients were on statin therapy before study entry, and about 50% of participants had been previously enrolled in the 4S trial. The primary endpoint was coronary death, nonfatal MI, or resuscitated cardiac arrest. IDEAL enrolled 8888 patients and had a mean follow-up period of 4.8 years. During follow-up, the mean LDL-C level was 104 mg/dL (2.69 mmol/L) in the 20-mg/40-mg simvastatin group and 82 mg/dL (2.12 mmol/L) in the 80-mg atorvastatin group. Major coronary events tended to be lower with intensive therapy (HR, 0.89; P = 0.07) but did not achieve statistical significance. Major cardiovascular events and any coronary event were significantly reduced by 13% (P = 0.02) and 16% (P < 0.001), respectively, in the 80-mg atorvastatin group. There was no excess risk of noncardiovascular death with intensive therapy (HR, 0.92; P = 0.47). With the exception of transient elevations in liver transaminase levels, there were no significant differences between treatments. The results of the TNT and IDEAL trials further established the important role for intensive statin therapy in the management of patients with stable CAD and extended the observations from PROVE IT-TIMI 22 in ACS patients to patients with stable disease. The IDEAL trial disproved the earlier concerns raised by TNT that intensive statin therapy might be associated with an increased risk of noncardiovascular mortality and provided further safety data on 80 mg of atorvastatin in more than 20,000 patient-years of follow-up. Some observers have questioned the importance of TNT and IDEAL, citing that the benefit of intensive therapy in these trials tended to be driven by the so-called soft endpoints, such as recurrent MI or revascularization, compared, for instance, with the landmark 4S trial, in which total mortality was reduced in the statin group compared with placebo. However, in the decade since 4S was completed, the management of CAD has improved dramatically with greater use of additional cardioprotective medication and greater use of revascularization. It is therefore unlikely that a significant benefit in all-cause mortality would be observed with intensive therapy compared with standard therapy unless a much larger trial (perhaps requiring about 50,000 patients) with a longer follow-up is conducted.
SEARCH Trial
The Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) trial is the largest and the most recent of the trials in stable coronary disease and randomized 12,064 participants to simvastatin 20 mg versus 80 mg and accrued events over 6.7 years of follow-up.32 The trial had a lower thereshold of total cholesterol (TC) such that individuals had to have a total cholesterol of at least 3.5 mmol/L if they were on statin therapy or 4.5 mmol/L if they were statin naive. At study entry baseline TC was 4.23 mmol/L and LDL-C was 2.50 mmol/L. The average LDL-C difference between more versus less intensive therapy was 0.35mmol/L, which resulted in a nonsignificant 6% reduction in any major vascular event (HR, 0.94; CI 0.88–1.01). Similarly, there were no significant reductions in stroke, coronary death, or coronary revascularizations. The only endpoint to show significant benefit was nonfatal MI, where a 1.1% absolute reduction in risk was observed (7.7% vs. 6.6%).
 Meta-Analysis of Intensive versus Standard Therapy
Meta-Analysis of Intensive versus Standard Therapy
The four intensive versus standard therapy trials used different endpoints to assess clinical benefit and were each underpowered to assess the historical endpoint of CAD death or nonfatal MI. A literature-based meta-analysis was conducted to obtain consistent large-scale evidence across trials. All eligible trials were required to have at least 1000 participants and a treatment duration of at least 2 years.33 The four trials discussed previously—PROVE IT-TIMI 22, A to Z, TNT, and IDEAL—were identified, providing information on 27,548 patients and approximately 120,000 patient-years of follow-up data.26,27,30,31 A separate meta-analysis of the same four trials also assessed the effect of intensive versus standard therapy for reductions in hospitalization with heart failure.34 The average, pooled baseline LDL-C level in the four trials was 130 mg/dL (3.3 mmol/L), which was reduced on average to 101 mg/dL (2.59 mmol/L). With intensive therapy, the average LDL-C level was lowered further to 75 mg/dL (1.92 mmol/L).33 This additional reduction in LDL-C was associated with a 16% reduction in the risk of CAD death or MI (OR, 0.84; P = 0.00003). Similarly, there was a reduction in the risk of any major cardiovascular event by 16% (P < 0.0001). There was a favorable trend toward reduction in CAD death (OR, 0.88; P = 0.054) and no excess risk in noncardiovascular mortality was observed (OR, 1.03; P = 0.73). Reductions were observed for stroke (OR, 0.82; P = 0.012) and for hospitalization for CHF (OR, 0.73; P < 0.001).34 The modest effects overall on all-cause mortality may, in part, reflect contemporary background therapy and also the differential risk between patients with stable CAD and ACS. There is clear evidence of an all-cause mortality benefit within 2 years of ACS from an individual participant meta-analysis of the PROVE IT-TIMI 22 and the A to Z trials.35 Over approximately 20,000 person-years worth of follow-up intensive statin therapy reduced all-cause mortality by 23% (HR, 0.77; CI 0.63–0.95) and in absolute terms by 1.3% (NNT, 77 over 2 years to prevent one death from any cause) from 4.9% to 3.6%. Statin therapy is now recommended for all patients with established atherosclerotic vascular disease. This meta-analysis extends the earlier findings (i.e., CTT meta-analysis) and demonstrates that beyond standard therapy, additional intensive LDL-C reduction provides a further 16% reduction in risk of CAD or nonfatal MI or any major cardiovascular event, or approximately a 50% reduction compared with placebo.
Given the improvement in standards of medical care and the use of revascularization over the past decade, these additional benefits may seem modest but are achieved over 2 to 5 years. Given the chronic, lifelong nature of these diseases, these benefits throughout an individual’s life would be expected to translate into greater absolute benefits by preventing recurrent, multiple events. In addition analytical techniques that take only time to first event into account underestimate the overall benefits of any treatment regimen; as therapies reduce the second and third events among those on more intensive treatments, the real benefit and cost effectiveness of more intensive regimens is even greater. For instance, in the PROVE IT-TIMI 22 trial over 2 years for every 2000 individuals treated, an additional 65 events could be averted by intensive therapy (P = 0.009).36 Similar consistent observations were made in the TNT trial in stable CAD where beyond the first event intensive statin therapy for approximately every 5000 individuals treated for 5 years resulted in 166 fewer second events, 92 fewer third events, 55 fewer fourth events, and 33 fewer fifth events.37 Taken together, these data support the value of long-term atheroprotection with intensive lipid lowering over time and the need for continuation of therapy.
More recently, the CTT in their second round of analyses provided individual participant data on five trials and 39,612 individuals.38
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree