Essentials of Diagnosis
General Considerations
Multiple epidemiologic studies have demonstrated the relationship between cardiovascular mortality and elevated plasma cholesterol levels. Lipid-lowering therapy, particularly with hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins), has demonstrated survival benefit in both primary prevention (patients without evidence of atherosclerotic cardiovascular disease [ASCVD]) and secondary prevention (patients with evidence of ASCVD). As a result, screening, risk stratifying, and treating dyslipidemia have become integral parts of preventive cardiovascular medicine.
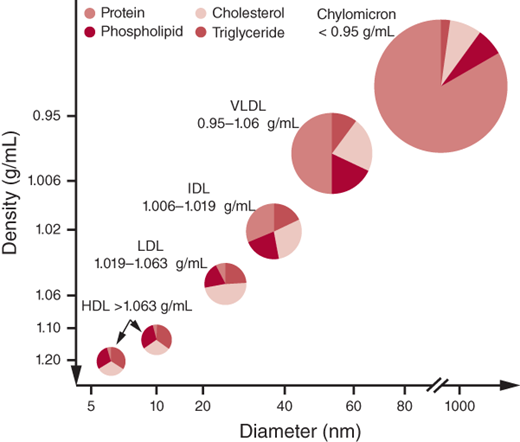
Cholesterol, cholesteryl esters, and triglycerides are the major lipids found in plasma. Cholesterol is an integral component of the cell membrane. It also serves a role in steroid hormone and bile acid synthesis. Triglycerides consist of fatty acid chains and phospholipids. Fatty acid chains are a primary source of energy in humans; phospholipids are key elements of all cell membranes. Cholesterol and triglycerides are insoluble in water and are transported in plasma by lipoproteins, classified as high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, very-low-density lipoprotein (VLDL) cholesterol, intermediate-density lipoprotein (IDL) cholesterol, and chylomicrons. Lipoproteins consist of a lipid core and a water-soluble phospholipid outer layer that carry apolipoproteins, which are specific proteins that serve as coenzymes or receptor ligands or act as regulators of lipoprotein metabolism. Apolipoprotein (apo) A-I is present in HDL and promotes cholesterol efflux from tissues. Apo B, present as either apo B-100 (VLDL, IDL, LDL) or apo B-48 (chylomicron), serves as the LDL receptor ligand. Apo C-I, apo C-II, and apo C-III participate in triglyceride metabolism, and apo E is present on chylomicrons.
Lipoprotein(a) [Lp(a)] highly correlates with cardiovascular disease (CVD) and consists of an LDL particle and a specific apolipoprotein A. The structure of Lp(a) is similar to plasminogen and is thought to increase thrombogenesis. It is known to predict early atherosclerotic risk independent of other risk factors. Figure 1–1 illustrates the classes of lipoproteins and their characteristics.
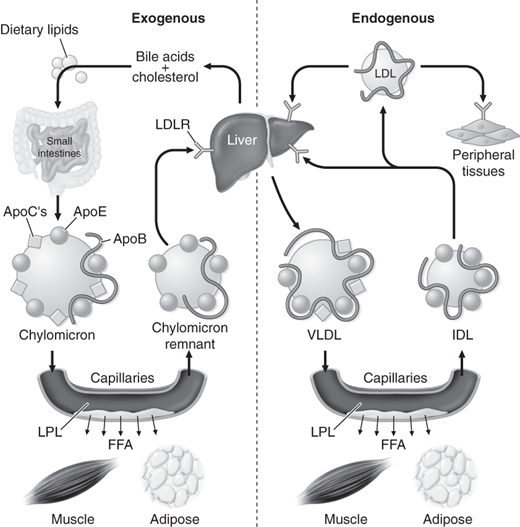
Transportation of dietary lipids to peripheral tissues and the liver is known as the exogenous metabolic pathway. Here, triglycerides are hydrolyzed by pancreatic lipases and are emulsified by bile acids, leading to micelle formation. Micelle uptake occurs in the intestinal brush border where fatty acids are re-esterified and packaged together with apo B-48, phospholipids, and cholesterol to form chylomicrons. Chylomicrons then reach the portal circulation where they are hydrolyzed to release fatty acids by lipoprotein lipase (LPL). LPL activity is regulated through apo C-II, which activates LPL, and apo C-III, which inhibits LPL. The free fatty acids are used by muscle or stored as fat in an insulin-regulated process. As the particles get smaller, the cholesterol and apolipoproteins are transferred to HDL, creating a chylomicron remnant that is then taken up by the liver for degradation in an apo E–mediated process.
The endogenous metabolic pathway refers to transport of hepatic lipoproteins to peripheral tissues via LDL. It ensures a steady supply of substrate given that food supply varies. Here VLDL, a triglyceride-rich lipoprotein, serves a similar role to the chylomicron in the exogenous pathway. VLDL is derived from esterified long-chain fatty acids in the liver. Its triglyceride component is also hydrolyzed by LPL. VLDL acquires apolipoproteins (B-100, C-I, C-II, C-III, and E) from HDL and also exchanges triglycerides for cholesteryl esters in a process mediated by cholesteryl ester transfer protein (CETP). This mechanism allows cholesterol transfer from HDL to VLDL, allowing reverse cholesterol transport. Following hydrolysis, up to 40% of the resulting VLDL remnant, known as IDL, is taken up by the liver in an apo E–mediated transfer, and the remainder is converted to LDL by hepatic lipase. LDL particles mostly contain cholesteryl esters together with apo B-100 and are the main carriers of cholesterol. LDL is internalized through apo B-100, which binds to LDL receptors present on the cell surface. Cells are also capable of synthesizing cholesterol through an enzymatic process that involves HMG-CoA reductase. Figure 1–2 illustrates the endogenous and exogenous lipid metabolism pathways.
Figure 1–2.
Endogenous and exogenous pathways of lipid metabolism. FFA, free fatty acids; IDL, intermediate-density lipoproteins; LDL, low-density lipoproteins; LDLR, low-density lipoprotein receptor; LPL, lipoprotein lipase; VLDL, very-low-density lipoproteins. (Reproduced with permission from Longo DL, et al. Harrison’s Principles of Internal Medicine, 18th ed. New York: McGraw-Hill; 2012.)
There is an inverse relationship between HDL levels and atherosclerotic coronary artery disease (CAD). HDL is responsible for reverse cholesterol transport. It inhibits lipoprotein oxidation and maintains endothelial integrity. Synthesized mostly in the liver but also in the intestine, the nascent HDL molecule consists of apo A-I, phospholipids, and cholesterol. HDL acquires cholesterol in an apo A-I–mediated process, which is then esterified by lecithin-cholesterol acyltransferase (LCAT) within the HDL particle forming mature HDL-containing cholesteryl esters, which can be taken up by the liver. Cholesterol and triglycerides can also be transferred from HDL to VLDL and chylomicrons via CETP, a process that allows HDL to transport cholesterol between cells, VLDL and chylomicrons, and the liver.
Dyslipidemia is a major predisposing factor in the development of atherosclerosis and ASCVD. Elevated plasma LDL concentrations can directly and indirectly contribute to atherogenesis. Oxidized LDL accumulates in subintimal macrophages through active scavenger receptor–mediated uptake that leads to cellular dysfunction, apoptosis, and necrosis. Unlike the LDL receptor–mediated endocytosis, this process is not regulated by cholesterol content. These macrophages are termed foam cells and form subintimal fatty streaks. This culminates in the release of proinflammatory and prothrombotic molecules, which causes endothelial injury. Oxidized LDL can also directly cause endothelial injury through interaction with the cell surface. Endothelial injury results in platelet aggregation and smooth muscle proliferation, which initiates atherosclerotic plaque formation. HDL serves a protective role in atherogenesis. Its antiatherogenic effects are exerted through maintenance of endothelial function, apo A-I–mediated reverse transport of macrophage cholesterol, antioxidant effects, and antithrombotic effects.
Clinical Findings
Patients with dyslipidemia are asymptomatic with the exception of those who present with acute pancreatitis in the setting of severe hypertriglyceridemia. The clinical findings depend on the cause of dyslipidemia.
The most severe forms of hyperlipidemia are caused by genetic disorders of lipoprotein metabolism such as familial hypercholesterolemia (FH). FH is an autosomal dominant disease with defects in the gene for the LDL receptor. The mutant gene prevents the LDL receptor from removing LDLs from the blood. LDL cholesterol levels are typically in excess of 300 mg/dL. Common manifestations include fatty skin deposits called xanthomas over parts of the hands, elbows, knees, and ankles and around the corner of the eye (xanthelasmas). It frequently presents as premature CAD with chest pain (angina) or acute myocardial infarction. Other common primary causes of dyslipidemia are familial combined hyperlipidemia (both high LDL and triglyceride levels) and pure hypertriglyceridemia (very high triglyceride levels).
Genetic Disorder | Protein (gene) Defect | Lipoproteins Elevated | Clinical Findings | Genetic Transmission | Estimated Incidence |
|---|---|---|---|---|---|
Lipoprotein lipase deficiency | LPL | Chylomicrons | Eruptive xanthomas, hepatosplenomegaly, pancreatitis | AR | 1 in 1,000,000 |
Familial apolipoprotein C-II deficiency | Ape-CII | Chylomicrons | Eruptive xanthomas, hepatosplenomegaly, pancreatitis | AR | < 1 in 1,000,000 |
Apo A-V deficiency | ApeA-V | Chylomicrons, VLDL | Eruptive xanthomas, hepatosplenomegaly, pancreatitis | AD | < 1 in 1,000,000 |
GP1HBP1 deficiency | GP1HBP1 | Chylomicrons | Eruptive xanthomas, pancreatitis | AD | < 1 in 1,000,000 |
Familial hepatic lipase deficiency | Hepatic lipase | VLDL remnants | Pancreatitis, CHD | AR | < 1 in 1,000,000 |
Familial dysbetalipoproteinemia | ApoE | Chylomicrons, VLDL remnants | Palmar and tuboeruptive xanthomas, CHD, PVD | AR, AD | 1 in 10,000 |
Familial hypercholesterolemia | LDL receptor | LDL | Tendon xantnomas, CHD | AD | 1 in 500 |
Familial defective apo B-l00 | ApoB-100 | LDL | Tendon xanthomas, CHD | AD | < 1 in 1000 |
Autosomal dominant hypercholesterolemia | PCSK9 | LDL | Tendon xantnomas, CHD | AD | < 1 in 1,000,000 |
Autosomal recessive hypercholesterolemia | LDLRAP | LDL |