fraction of 10%, or multiple areas of ischemia is a class I indication for catheterization. These indications hold true even if the patient is asymptomatic. Positive stress tests without high-risk criteria are class II indications for LHC.
TABLE 64.1 Indications and Contraindications to Left Heart Catheterization | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
of the patient’s nameplate. The usual frame rate of cine film is set at 30 frames/s; 60 frames/s can be useful for patients with tachycardia. In thin individuals who are bradycardic (< 60 beats/min), the frame rate can be lowered to 15 frames/s. In addition, the table should move freely to the level of the patient’s groin.
radiation exposure. Leaded eyeglasses should also be considered. In addition, radiation badges are worn inside the lead apron and outside the thyroid collar to monitor cumulative radiation exposure. A leaded acrylic shield should be used between the patient and the operator closest to the patient. Standing further from the table also reduces radiation exposure by the inverse square of the distance. A number of additional steps can be taken to minimize radiation to both the operator and the patient. Fluoroscopy and, in particular, cine time should be minimized. The image intensifier should be positioned as close as possible to the patient to reduce radiation scatter. To decrease radiation, higher magnification should be used judiciously. “Coning down” on a region of interest with the use of collimators can also reduce the amount of radiation, as can the use of lung field collimators. Right anterior oblique (RAO) views produce less radiation scatter for the operator than left anterior oblique (LAO) views. Higher cine frame rates increase radiation exposure; use of 15 or 30 frames/s produces less radiation exposure than use of 60 frames/s. In the rare situation that a pregnant patient needs catheterization, a lead apron should be used. This precaution should also be taken for premenopausal women.
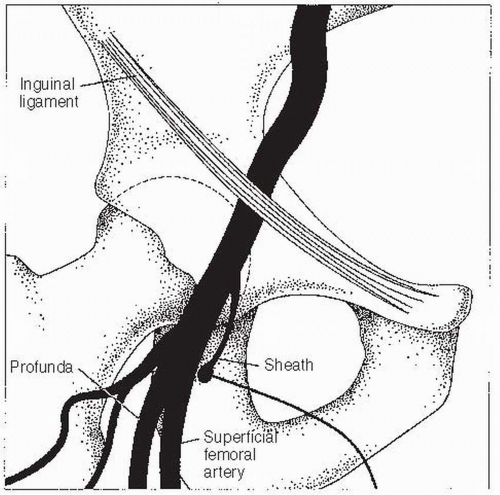
Alternatively, fluoroscopy can be used to locate the femoral head. The entry point on the skin is located over the inferior border of the femoral head. Care must be taken not to enter the artery above the inguinal ligament, as this increases the chance of retroperitoneal bleeding. Arterial entry that is too low must also be avoided, as this can lead to pseudoaneurysm or arteriovenous fistula formation. Once the site of entry has been identified (and marked, if so desired), the area is cleaned with povidone iodine (Betadine) and surgically draped. Local anesthesia is given slowly (it hurts less when delivered slowly) while the clinician monitors the HR and watches for signs of a vagal reaction (nausea, lightheadedness, and yawning). The usual choice is procaine 1%. A subcutaneous wheal is raised with about 3 mL using a 25G needle. Next, an additional 6 to 10 mL is delivered to the deeper tissues with a 22G needle. In patients who are allergic to ester-type anesthetics, lidocaine 2% can be used. Once the site is anesthetized, an 18G Cook needle is inserted into the artery. Upon nearing the artery, a side-to-side motion of the needle indicates a position either medial or lateral to the artery. Up-and-down motion indicates correct positioning. In addition, when the needle is above the artery, it transmits the arterial pulsation to the fingertips. Once brisk arterial blood return is established, a 0.035” J-tipped 45-cm guidewire is inserted, the needle is withdrawn, and an arterial sheath with a dilator is placed over the wire. Then the wire and dilator are removed. The sheath is then flushed with saline. A 5F or 6F sheath is generally used for diagnostic catheterizations in the United States, though 4F sheaths are often used in Europe. An 8F sheath is used for acute cases or planned interventions. A 5F sheath is preferred over larger sheaths for patients with peripheral vascular disease.
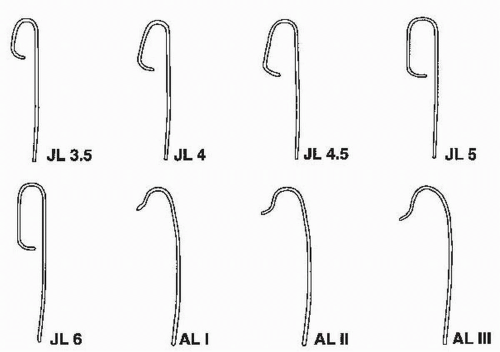 Figure 64.2 Catheters used for cannulating the left coronary artery. JL, Judkins left; AL, Amplatz left. |
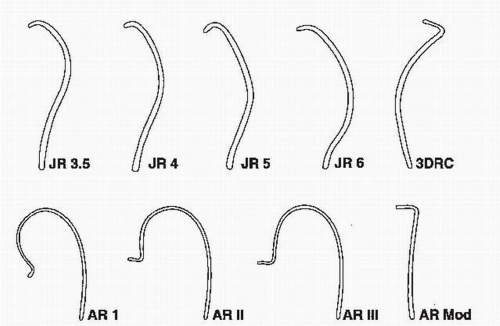 FIGURE 64.3 Catheters used for cannulating the right coronary artery. JR, Judkins right; AR, Amplatz right; 3DRC, no torque right coronary catheter; Mod, modified. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree