Because left atrial (LA) volume plays a critical role in determining cardiovascular outcomes, it was hypothesized that this might be related to the distensibility of the left atrium and how this relates to left ventricular filling pressure (LVFP). Echocardiographic estimates of LVFP were compared to cardiac catheterization measurements in 521 consecutive patients with acute myocardial infarction and correlated with short- and long-term outcomes. Receiver-operating characteristic curve analysis was performed to investigate the sensitivity and specificity of echocardiographic parameters for predicting elevated LVFP (>15 mm Hg). LA distensibility was calculated as (maximal volume − minimal volume) × 100%/minimal volume . and was found to be logarithmically associated with LVFP (p <0.0001). LA distensibility was superior to mitral E/annular Em for identifying increased LVFP (area under the receiver-operating characteristic curve 0.92 vs 0.78). A total of 44 patients died during hospitalization, and 89 patients had died or experienced heart failure requiring rehospitalization at 12-month follow-up. In a multivariate Cox regression model, LA distensibility was an independent predictor of in-hospital mortality (hazard ratio 2.373 for LA distensibility ≤60%, p = 0.026), while LA volume was an independent prognostic factor of 1-year death or heart failure (hazard ratio 2.266 for LA volume ≥34 ml/m 2 , p = 0.007). In conclusion, LA distensibility accurately identifies patients with increased LVFP after acute myocardial infarction and is an independent predictor of in-hospital mortality.
Left atrial (LA) volume provides significant prognostic information in the general population and in patients with heart disease, including acute myocardial infarction (AMI), left ventricular dysfunction, mitral regurgitation, cardiomyopathy, and atrial fibrillation. A large LA volume in any of these clinical scenarios indicates chronic diastolic dysfunction and is associated with poor outcomes, irrespective of systolic function. Although LA volume would not be expected to correlate with acute measurements of left ventricular filling pressure (LVFP), it is possible that dynamic changes in LA volume throughout the cardiac cycle (LA distensibility) might identify when LA and left ventricular pressures are acutely elevated. In this context, as LA pressure increases to maintain adequate left ventricular diastolic filling, atrial wall tension increases, leading to reduced distensibility. The high frame rate of echocardiography allows the measurement of these dynamic changes in LA volume. Because elevated LVFP after AMI has been associated with early adverse outcomes, reduced LA distensibility measured with echocardiography might be useful for identifying elevated LVFP noninvasively. This could provide critical bedside information, which would alter early management and identify patients at risk for increased complications. The objectives of the present study were (1) to evaluate the relation between LA volume parameters (including LA distensibility) and LVFP in patients with AMI and (2) to assess the prognostic value of LA volume measurements, compared to tissue Doppler imaging (TDI) parameters, in predicting the cardiac events in patients with AMI.
Methods
This study was approved by the institutional review board at Kaohsiung Veterans General Hospital. From December 2007 to May 2009, we enrolled 642 consecutive patients with AMI who had undergone cardiac catheterization for potential primary percutaneous coronary intervention (PCI). AMI was defined according to the European Society of Cardiology and American College of Cardiology guidelines, which require the presence of ≥2 of the following criteria: chest pain lasting >30 minutes, typical electrocardiographic changes, and serial elevations in the creatine kinase-MB fraction.
Myocardial infarction location was determined by electrocardiographic criteria. Exclusion criteria were (1) the presence of significant valvular heart disease, (2) any abnormality of the interatrial septum that precluded planimetry (e.g., atrial septal defect, aneurysm), (3) rhythm other than sinus, (4) inadequate image quality, and (5) a lack of informed consent. Patients requiring emergent coronary bypass grafting and/or repair for cardiac rupture or ventricular septal defect were excluded. Ultimately, 521 patients were enrolled for the final analysis. Single-vessel disease was defined as no other stenosis >70% over nonculprit vascular territories. Otherwise, any stenosis >70% in the nonculprit vascular territory classified a patient as having multivessel disease. Renal dysfunction was defined as estimated creatinine clearance <60 ml/min in the first blood sample in the emergent department.
All patients gave written informed consent to participate in the study. Primary PCI and stenting were performed for just the culprit lesion using standard techniques. The measurements of LVFP were performed after coronary angiography if PCI was not indicated or after primary PCI (no patient underwent left ventriculography). The fourth intercostal space in the midaxillary line was used as the zero level. LVFP was continuously recorded (50 mm/s) by a 6Fr pigtail catheter placed at the mid left ventricular cavity using fluoroscopic screening. Digital records of rapid-acquisition left ventricular pressure tracings were recorded. Measurements were made offline from the digitized recordings. The average value of pre-A pressure over 5 cardiac cycles was used as LVFP. LVFP >15 mm Hg was considered elevated.
Echocardiography was performed immediately after LVFP measurements while patients were still in the catheterization laboratory. Slight adjustments were made in patient position to optimize acquisition of apical windows. In the apical views, a pulsed-wave Doppler sample volume was placed at the level of the mitral annulus over the septal and lateral borders. Pulsed-wave TDI results were characterized by a myocardial systolic wave (Sm) and 2 diastolic waves: early (Em) and atrial contraction (Am). The pulsed-wave TDI tracing was recorded over 5 cardiac cycles at a sweep speed of 100 mm/s and was used for offline calculations. The average Em of septal and lateral mitral annulus was chosen to estimate LVFP using the E/Em method.
All volume measurements were calculated from apical 4- and 2-chamber views using the biplane area-length method. LA volumes were measured at 2 points: (1) immediately before the mitral valve opening (maximal LA volume) and (2) at the mitral valve closure (minimal LA volume). LA distensibility was calculated as (maximal volume − minimal volume) × 100%/minimal volume. In all patients, LA volumes were indexed to body surface area. Figure 1 demonstrates the measurements of LA distensibility.
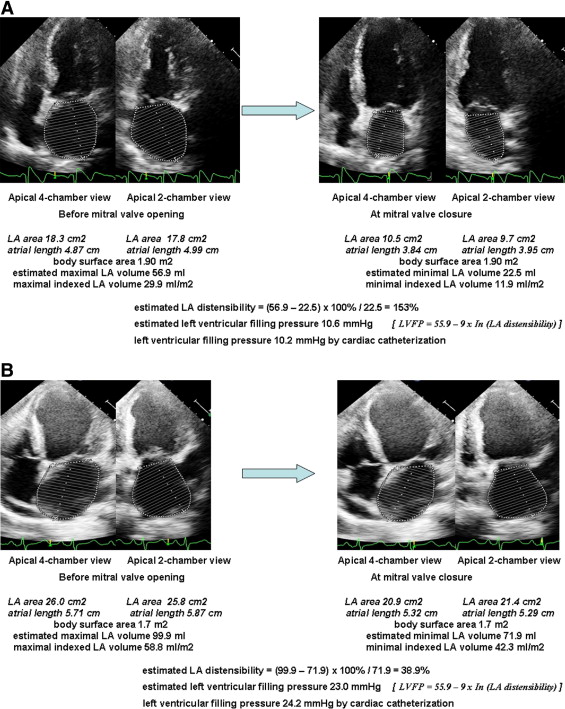
During the index hospitalization, only in-hospital death was deemed an event. After the index hospitalization, patients were followed up at our cardiovascular clinic for ≥1 year. A follow-up survey assessing hard cardiovascular events was carried out after discharge. All-cause mortality and rehospitalization for congestive heart failure were defined as hard cardiovascular events. Heart failure with rehospitalization was defined as ≥1 of the following criteria: (1) worsening exercise tolerance and respiratory distress with New York Heart Association class III or IV symptoms or (2) the presence of pulmonary rales or chest radiography showing pulmonary congestion requiring oral or intravenous diuretics during an in-hospital stay. In patients with multivessel disease who had undergone primary PCI for culprit lesions, scheduled interventions (either PCI or coronary artery bypass grafting) for nonculprit lesions were not considered hard cardiovascular events. Follow-up was performed from December 2007 to June 2010 by telephone interviews, medical record reviews, and home visits. No patient was lost to follow-up during the time period.
In the first 50 enrolled cases, maximal LA volume and minimal LA volume were measured by 2 independent observers. Interobserver variability was calculated as the difference between the values obtained by the 2 observers divided by the mean. Interobserver differences and variability were 4.1 ± 5.4 ml and 6.6 ± 8.7% for maximal volume and 2.9 ± 3.2 ml and 8.1 ± 8.9% for minimal volume, respectively. Therefore, interobserver variability in LA distensibility was 7.8 ± 6.6%.
SPSS (SPSS, Inc., Chicago, Illinois) was used for all statistical analyses. Baseline characteristics and echocardiographic parameters of the study patients were analyzed according to events (in-hospital mortality and 1-year hard cardiovascular events). All continuous variables are expressed as mean ± SD. A p value <0.05 was considered statistically significant. Comparison of clinical characteristics was performed using chi-square analysis for categorical variables. Univariate and multivariate logistic regression analyses were performed on potential variables for predictions of events. Variables considered potential predictors for multivariate modeling were selected by univariate analyses and subsequently selected with entry and retention in the model set at a significance level of 0.05. Otherwise, other known risk predictors (e.g., renal dysfunction, multivessel disease, and E/Em) were also entered in the model. To evaluate the effect of covariates on in-hospital mortality and hard cardiovascular event, relative risk and 95% confidence intervals were calculated as hazard ratios derived from the Cox proportional-hazards model. Receiver-operating characteristic curve analysis was also performed to assess the sensitivity and specificity for predicting elevated LVFP (>15 mm Hg).
Results
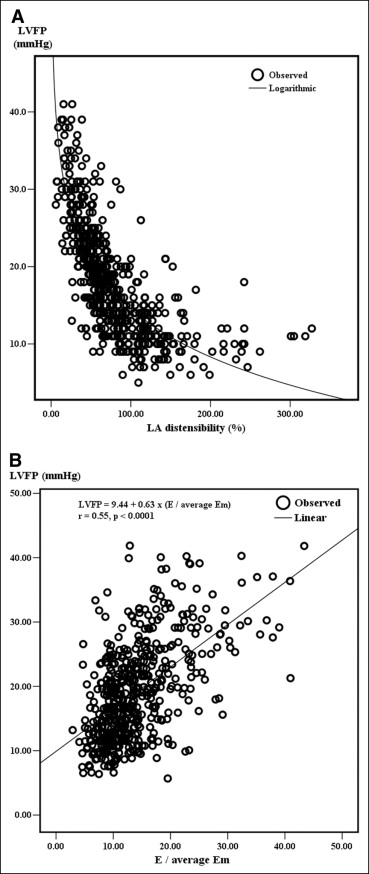
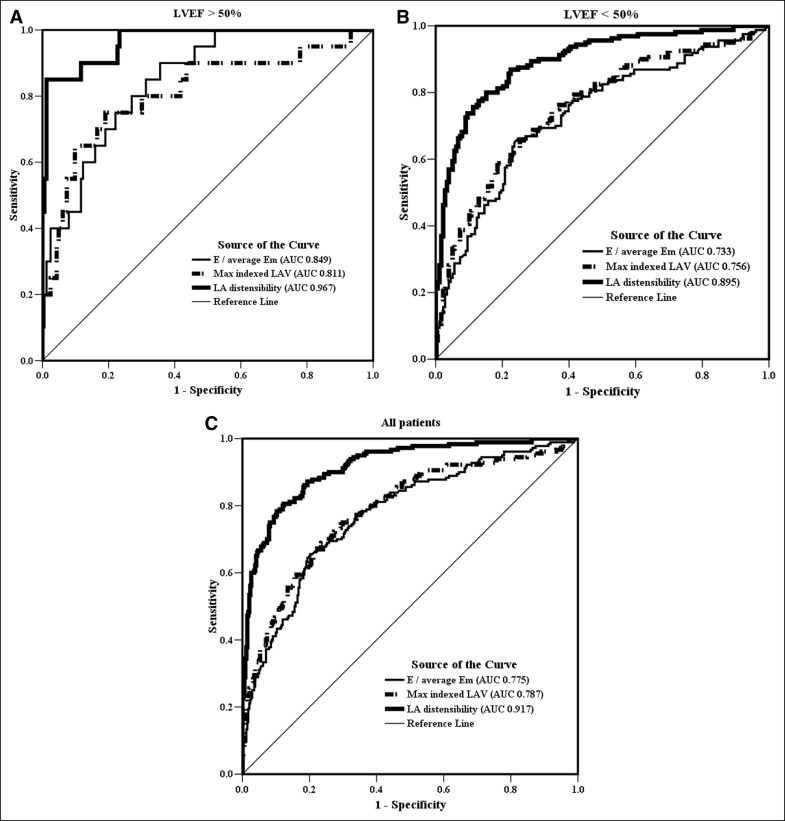
Table 1 lists the basic characteristics of all enrolled patients. The interval between symptom onset and catheterization was 4.1 ± 5.2 hours (range 0.8 to 11.8). A total of 44 patients (8.4%) died during the index hospitalization, and 89 of 521 patients (17.1%) had experienced hard cardiovascular events at 1-year follow-up. Table 2 lists the correlations between LA volume parameters, E/Em, and LVFP. LA distensibility was correlated inversely with LVFP and directly with the severity of LV systolic dysfunction. The correlation with LVFP was logarithmic (LVFP = 55.9 − 9 × ln[LA distensibility], F = 793.85, p <0.0001; Figure 2 ). E/Em was correlated linearly with LVFP (F = 225.24, p <0.0001; Figure 2 ). Subgroup analyses were performed. LA distensibility was correlated closely with LVFP independent of coronary status, the ejection fraction, and Killip class. LA distensibility was superior to E/Em and maximal indexed LA volume for predicting elevated LVFP, regardless of the left ventricular ejection fraction ( Figure 3 ). LA distensibility ≤60% had an area under the receiver-operating characteristic curve of 0.917 (sensitivity 86%, specificity 82%) for predicting high LVFP.
| Variable | Value |
|---|---|
| Age (years) | 65 ± 14 |
| Women/men | 107/414 |
| Hypertension | 316 (60.7%) |
| Diabetes mellitus | 188 (36.1%) |
| Current smoker | 271 (52.0%) |
| Previous myocardial infarction | 33 (6.3%) |
| Renal dysfunction | 291 (55.9%) |
| Total cholesterol (mg/dl) | 180 ± 44 |
| Low-density lipoprotein cholesterol (mg/dl) | 109 ± 34 |
| Heart rate (beats/min) | 77 ± 15 |
| Systolic blood pressure (mm Hg) | 112 ± 41 |
| Non–ST-segment elevation myocardial infarction | 128 (24.6%) |
| Location of ST-segment elevation myocardial infarction | |
| Anterior | 240 (46.1%) |
| Lateral | 19 (3.6%) |
| Inferior | 134 (25.7%) |
| Killip classification | |
| I | 211 (40.5%) |
| II | 178 (34.2%) |
| III | 109 (20.9%) |
| IV | 23 (4.4%) |
| Vascular status | |
| Single-vessel disease | 254 (48.8%) |
| Double-vessel disease | 98 (18.8%) |
| Triple-vessel disease | 169 (32.4%) |
| Peak creatinine kinase (U/L) | 2,053 ± 2104 |
| Respiratory failure | 87 (16.7%) |
| Intra-aortic balloon pump | 22 (4.2%) |
| LVFP (mm Hg) | 18.3 ± 7.5 |
| Hard cardiovascular event (death and heart failure) | 89 (17.1%) |
| In-hospital mortality | 44 (8.4%) |
| Condition | Pearson’s Correlation Between LVFP and Diastolic Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Maximal Indexed LA Volume | LA Distensibility | E/Septal Em | E/Lateral Em | E/Average Em | ||||||
| Correlation | p Value | Correlation | p Value | Correlation | p Value | Correlation | p Value | Correlation | p Value | |
| All patients (n = 521) | 0.533 | <0.0001 | −0.686 | <0.0001 | 0.544 | <0.0001 | 0.46 | <0.0001 | 0.543 | <0.0001 |
| LVEF >50% (n = 183) | 0.515 | <0.0001 | −0.606 | <0.0001 | 0.497 | <0.0001 | 0.454 | <0.0001 | 0.457 | <0.0001 |
| LVEF ≤50% (n = 338) | 0.499 | <0.0001 | −0.671 | <0.0001 | 0.512 | <0.0001 | 0.416 | <0.0001 | 0.516 | <0.0001 |
| Vascular status | ||||||||||
| Single-vessel disease (n = 254) | 0.512 | <0.0001 | −0.658 | <0.0001 | 0.409 | <0.0001 | 0.418 | <0.0001 | 0.464 | <0.0001 |
| Double-vessel disease (n = 98) | 0.556 | <0.0001 | −0.657 | <0.0001 | 0.478 | <0.0001 | 0.375 | <0.0001 | 0.47 | <0.0001 |
| Triple-vessel disease (n = 169) | 0.479 | <0.0001 | −0.734 | <0.0001 | 0.497 | <0.0001 | 0.441 | <0.0001 | 0.509 | <0.0001 |
| Killip class | ||||||||||
| I (n = 211) | 0.602 | <0.0001 | −0.586 | <0.0001 | 0.477 | <0.0001 | 0.347 | <0.0001 | 0.403 | <0.0001 |
| II (n = 178) | 0.652 | <0.0001 | −0.691 | <0.0001 | 0.477 | <0.0001 | 0.426 | <0.0001 | 0.53 | <0.0001 |
| III (n = 109) | 0.398 | <0.0001 | −0.606 | <0.0001 | 0.402 | <0.0001 | 0.366 | <0.0001 | 0.399 | <0.0001 |
| IV (n = 23) | 0.544 | 0.001 | −0.778 | <0.0001 | 0.66 | <0.0001 | 0.63 | <0.0001 | 0.693 | <0.0001 |
Univariate and multivariate associations with in-hospital mortality are listed in Table 3 . In the final model, the hazard ratio for a 10% decrease in LA distensibility was 1.167. In a Cox regression analysis with LA distensibility ≤60% as a categorical variable, the age-adjusted hazard ratio for in-hospital mortality, compared with that with LA distensibility >60%, was 3.247 (95% confidence interval 1.956 to 5.391, p <0.0001). In a multivariate Cox regression model, the hazard ratio was 2.373 (95% confidence interval 1.108 to 5.079, p = 0.026). The predictors of 1-year hard cardiovascular events by univariate and multivariate analysis are listed in Table 4 . In the final model, maximal indexed LA volume was associated with a hazard ratio of 1.014 for each 1 ml/m 2 increase in volume. LA distensibility, remote myocardial infarction, multivessel disease, and renal dysfunction were not significant predictors of 1-year hard cardiovascular events. The multivariate adjusted hazard ratio of 1-year hard cardiovascular events with maximal indexed LA volume ≥34 ml/m 2 as a categorical variable was 2.266 (95% confidence interval 1.246 to 4.122, p = 0.007).
| Variable | Survival (n = 477) | Mortality (n = 44) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Values | HR (95% CI) | p Value | |||
| Age (years) | 64 ± 14 | 75 ± 13 | 1.08 (1.03–1.13) | 0.002 | 1.053 (1.022–1.084) per year | 0.001 |
| Women/men | 92/385 | 15/29 | 1.47 (0.49–4.41) ⁎ | 0.495 | ||
| Hypertension | 281 (58.9%) | 35 (79.5%) | 2.53 (0.92–6.95) | 0.073 | ||
| Diabetes mellitus | 162 (34%) | 26 (59.1%) | 1.86 (0.75–4.63) | 0.184 | ||
| Current smoker | 263 (55.1%) | 22 (50%) | 2.26 (0.92–6.21) | 0.102 | ||
| Renal dysfunction | 253 (53%) | 38 (86.4%) | 1.02 (0.69–2.88) | 0.839 | 1.394 (0.518–3.752) | 0.51 |
| Previous myocardial infarction | 28 (5.9%) | 5 (11.4%) | 1.22 (0.71–2.28) | 0.427 | ||
| Heart rate (beats/min) | 76 ± 14 | 87 ± 18 | 1.04 (0.98–1.10) | 0.125 | ||
| Systolic blood pressure (mm Hg) | 117 ± 41 | 98 ± 36 | 0.94 (0.90–1.24) | 0.164 | ||
| Non–ST-segment elevation myocardial infarction | 105 (22%) | 23 (52.3%) | 2.81 (1.14–6.27) | 0.001 | 3.223 (1.590–6.530) | 0.001 |
| Killip class | 2.13 (1.21–3.76) † | 0.009 | 2.418 (1.600–3.654) per 1-class increase | <0.0001 | ||
| I | 207 (43.4%) | 4 (9.1%) | ||||
| II | 170 (35.6%) | 8 (18.2%) | ||||
| III | 83 (17.4%) | 26 (59.1%) | ||||
| IV | 17 (3.6%) | 6 (13.6%) | ||||
| Vascular status | 0.45 (0.25–0.81) ‡ | 0.008 | 0.677 (0.462–0.994) per 1-vessel increase | 0.046 | ||
| Single-vessel disease | 241 (50.5%) | 13 (29.5%) | ||||
| Double-vessel disease | 88 (18.4%) | 10 (22.7%) | ||||
| Triple-vessel disease | 148 (31%) | 21 (47.7%) | ||||
| Peak creatinine kinase (U/L) | 2,071 ± 2088 | 1,855 ± 2090 | 1.00 (1.00–1.01) § | 0.466 | ||
| Mitral E velocity (cm/s) | 77 ± 24 | 91 ± 24 | 0.99 (0.97–1.02) | 0.512 | ||
| Mitral A velocity (cm/s) | 76 ± 22 | 70 ± 27 | 0.98 (0.96–1.04) | 0.214 | ||
| Deceleration time (ms) | 190 ± 52 | 170 ± 50 | 1.01 (0.99–1.01) | 0.681 | ||
| Left ventricular ejection fraction (%) | 46 ± 9 | 37 ± 9 | 1.06 (1.01–1.13) ∥ | 0.039 | 1.056 (1.013–1.101) per 1% decrease | 0.01 |
| Maximal indexed LA volume (ml/m 2 ) | 34 ± 13 | 42 ± 13 | 1.08 (0.90–1.31) | 0.418 | 1.007 (0.985–1.029) per 1 ml/m 2 increase | 0.526 |
| Minimal indexed LA volume (ml/m 2 ) | 21 ± 11 | 30 ± 11 | 0.83 (0.59–1.16) | 0.279 | ||
| LA distensibility (%) | 84 ± 55% | 44 ± 26% | 1.15 (1.09–2.36) ¶ | 0.001 | 1.167 (1.018–1.338) per 10% decrease | 0.027 |
| Mitral E/average Em | 14 ± 6 | 18 ± 8 | 1.02 (0.95–1.10) | 0.519 | 0.992 (0.952–1.034) | 0.651 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


