47 Left Atrial Appendage Closure and Stroke
Local Device Therapy for Cardioembolic Stroke Protection
 Atrial Fibrillation and Stroke
Atrial Fibrillation and Stroke
AF is the most prevalent significant cardiac arrhythmia in the world. It affects more than two million people age 40 years or more (2.3% of the U.S. population).1–5 The median age of patients with AF is 75 years. Accordingly, as the population ages, the number of patients will increase drastically, and by the year 2020, more than 3,300,000 patients will have been affected. AF prevalence increases with age, 1.5% of those aged 50 to 59 years are affected; and nearly 24% of people aged 80 to 89 years have this arrhythmia.6 Roughly 500,000 ischemic strokes occur each year in the United States. AF is responsible for 20% of these strokes; among patients aged 50 to 59 years, 10% of ischemic strokes are caused by AF, whereas among patients aged 80 to 89 years, this number dramatically rises to 32%.6–8 Stroke is the number one cause of long-term disability and the third leading cause of death in patients with AF.4,9 Patients with AF have a fivefold increased stroke risk compared with the general population. Nearly 87% of ischemic strokes are thromboembolic, and of these, nearly 90% of emboli originate in the LAA. In nonvalvular AF, strokes can be catastrophic events.10 Henricksson examined stroke survival, and in 31,000 patients with AF in the Swedish Stroke Registry, the AF diagnosis carried a much higher death risk, even after adjustment for age and gender.11 Thirty-one percent of strokes in nonvalvular AF are fatal, 28% are seriously disabling, and 11% are moderately disabling10 (Fig. 47-1). Strokes in AF differ from those caused by vascular atheroemboli (e.g., atheroma in the carotid, the aorta, and vertebral arteries). Emboli originating from within the heart are more clinically serious; they carry higher mortality and often cause worse disability, possibly because intracardiac thrombi (from the LAA) are larger than embolic atheromatous vascular debris.9,12–14 Strokes from AF affect the brain parenchyma 25 times more often compared with strokes from carotid artery disease.15 Clinical symptoms involving the retina likely originate from carotid artery disease rather than from AF or cardioemboli. Patients presenting with retinal symptoms are, thus, far more likely to have a cerebrovascular cause rather than a cardioembolic cause.15 AF is associated with more subclinical brain infarcts, as shown in the VA SPINAF (Veterans Administration Stroke Prevention in Atrial Fibrillation) trial, in which 15% of patients with AF had subclinical strokes occurring at 1.3% per year. Interestingly, comparable subclinical stroke and transient ischemic attack (TIA) rates occur even in patients taking both aspirin and warfarin.16,17
 Warfarin
Warfarin
State of the Art for Stroke Prophylaxis in Atrial Fibrillation
Warfarin is the best medical therapy for stroke prevention in nonvalvular AF. Stroke risk in AF is assessed by the CHADS-2 score (See Table 47-1 for CHADS-2 score definition, and Table 47-2 for stroke risk with and without warfarin). Many well known, large trials agree on the benefits of warfarin, including the Stroke Prevention in Atrial Fibrillation (SPAF)- I, II, and III; the Canadian Atrial Fibrillation Anticoagulation (CAFA); the Boston Area Anticoagulation Trial for Atrial Fibrillation (BAATAF); and the SPINAF trials.18–23 Meta-analyses of these trials compare stroke prevention in nonvalvular AF using warfarin, aspirin, and placebo (see Table 47-3 for therapy recommendations based on these trials). The collective message is that warfarin is effective in reducing stroke and death compared with aspirin and placebo. When given in appropriate, therapeutic doses with therapeutic international normalized ratio (INR) values, warfarin reduces stroke risk by 60% to 70% compared with no treatment and by 30% to 40% compared with aspirin alone.15 Although warfarin is a therapeutic cornerstone, it has major limitations and has serious adverse effects. For example, in one large meta-analysis, the authors calculated that if 51 ischemic strokes occur per 1000 patient-years without warfarin treatment, appropriately dosed warfarin would be expected to prevent 28 strokes but at the expense of 11 fatal bleeds. Aspirin therapy alone would prevent 16 strokes at the expense of 6 fatal bleeds.24,25 Patients with AF taking neither warfarin nor aspirin have a stroke risk of 5% per year.
TABLE 47-1 CHADS-2 Score Calculation
| Condition | Points |
|---|---|
| Prior stroke | 2 |
| Congestive heart failure | 1 |
| Hypertension | 1 |
| Age >75 years | 1 |
| Diabetes | 1 |
TABLE 47-2 Annual Stroke Risk (percent/year) by CHADS-2 Score, with and without Warfarin Therapy
| CHADS | Warfarin | No Warfarin |
|---|---|---|
| 0 | 0.25 | 0.49 |
| 1 | 0.72 | 1.50 |
| 2 | 1.27 | 2.50 |
| 3 | 2.20 | 5.27 |
| 4 | 2.35 | 6.02 |
| 5 or 6 | 4.60 | 6.88 |
TABLE 47-3 Prophylaxis Recommendations based on Age and Clinical Status
| Age | Clinical Condition | Therapy |
|---|---|---|
| >60 years | Coronary artery disease (CAD) or diabetes | Warfarin |
| >75 years | All patients | Warfarin |
| All | Heart failure | Warfarin |
| All | Hypertension | Warfarin |
| All | Prior embolism | Warfarin |
| All | Thrombus present by trans-esophageal echocardiography (TEE) | Warfarin |
Warfarin is often difficult to control from the perspectives of both safety and efficacy. Bungard examined INR values in a population of AF patients taking warfarin in the emergency department. The mean age was 73 years, and 45% of these patients had subtherapeutic INR values, 37% had therapeutic values, and 19% had supratherapeutic values. Thus, even in warfarin-treated patients, 63% were out of the prescribed INR range.26 Another study examined combined patients from the Stroke Prevention using ORal Thrombin Inhibitor in atrial Fibrillation (SPORTIF) III and V trials, who were taking warfarin, and divided their INR control into groups rated as poor, moderate, or good (therapeutic INR <60%, 60% to 75%, or >75% of the time, respectively). Only 60% of patients taking warfarin were within a therapeutic INR range (2–3.0), 29% had INR levels below 2, and 15% had levels above 3. Patients with poorly controlled disease (INR too high) compared with those with moderate or good control have much higher mortality (4.2% vs. 1.8% and 1.7% per year, respectively) and major bleeding (3.9% vs. 2% and 1.6%). Patients with poorly controlled disease have significantly higher strokes and systemic embolization compared with those with good control (2.1% vs. 1.1%).27 Warfarin effectiveness is impacted by its interactions with food, other medications, and ill-defined genetically determined responses. It has a long half-life and is a problem in patients undergoing percutaneous coronary interventions (PCIs), since triple therapy—with acetyl salicylic acid (ASA), clopidogrel, and warfarin—is mandated in this situation, even though it is associated with a rate of bleeds as high as 9% per year. Perhaps because of all these considerations, as well as patient intolerance, noncompliance, or other management difficulties, less than 50% of patients who need warfarin and are eligible to take it are treated at therapeutic INR levels. Safety is a clear concern, since hemorrhagic stroke in patients taking warfarin is very often fatal. These concerns may underlie warfarin under-utilization in AF. For example, one study found that only 55% of patients with AF and no contraindications actually took the drug within 3 months preceding the study; other studies cite even lower warfarin use in these patients, ranging between 17% and 50%.28 Older patients have the highest absolute stroke risk in AF and yet are the least likely to take warfarin, as contraindications to warfarin are present in 30% to 40% of them.
Hemorrhage in Patients Taking Warfarin
While warfarin is better at stroke prevention compared with placebo or aspirin, intracerebral hemorrhage is a substantial risk in warfarin-anticoagulated patients.28a,28b Warfarin was evaluated using a meta-analysis of 28,044 patients with AF (from 29 centers) who were followed up for a mean of 1.5 years.29 Though warfarin limited stroke by 64% (aspirin by 22%), the rate of intracranial hemorrhage doubled in the warfarin group compared with the aspirin group. Another study also examined major hemorrhage during the first year of warfarin therapy in older patients with AF.30 In 492 of these patients (>65 years old), major bleeding (including intracranial) during the first year of treatment was 7%.29 The risk of intracranial bleeding increases with age.31
 Other Pharmacologic Therapies for Stroke Prevention
Other Pharmacologic Therapies for Stroke Prevention
Combined anti-platelet (aspirin plus clopidogrel) therapy is not as effective as warfarin for preventing stroke. The Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE) W trial reported on AF and stroke in patients randomized to warfarin or aspirin plus clopidogrel.32 Warfarin was substantially superior to anti-platelet therapy, and the trial was terminated before its planned follow-up. New therapies under consideration for stroke prophylaxis in AF include direct oral thrombin or Factor Xa inhibitors.33 Dabigatran is one such oral thrombin inhibitor recently approved by the U.S. Food and Drug Administration (FDA). In the Randomised Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial, 18,113 randomized patients with nonvalvular AF were followed for primary outcomes defined as stroke or systemic embolism.34 Two doses of dabigatran (110 mg or 150 mg orally twice daily) were given to patients and compared with standard-care warfarin after a 2-year follow-up. Primary outcomes occurred at 1.7% per year in the warfarin group versus 1.5% per year in the dabigatran 110 mg and 1.1% per year in the dabigatran 150 mg groups. Major bleeds occurred in 3.4% per year with warfarin versus 2.7% per year and 3.1% per year in the dabigatran 110 mg and 150 mg groups, respectively. Warfarin caused hemorrhagic stroke in 0.38% per year versus 0.1% in both dabigatran groups, whereas mortality was 4.1% per year for warfarin versus 3.4% and 3.6% per year in the dabigatran 110 mg and 150 mg groups. The study may be summarized as follows: oral dabigatran 110 mg twice daily has stroke and embolism rates similar to those with warfarin but less probability of bleeds, whereas dabigatran 150 twice daily has lower stroke and systemic embolism rates but similar hemorrhage rates. Also, more myocardial infarctions (MIs) occurred in the dabigatran 150 group compared with the warfarin group (0.74% vs. 0.53%, P = NS), a finding of unclear safety significance. It will be difficult to determine the optimal dose, as the ability to predict stroke is limited despite using predictive scores. A new bleeding risk score (HAS-BLED) may assist in this capacity:34a Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly (>65), Drugs and alcohol concomitantly.36 Based on many of these considerations, an alternative technology for stroke prevention in AF that is both safe and effective is clearly needed. The oral anticoagulant strategy amounts to a systemic treatment for what is a local problem, namely, thrombus formation within the LAA.
 Left Atrial Appendage
Left Atrial Appendage
Embryology, Structure, and Function
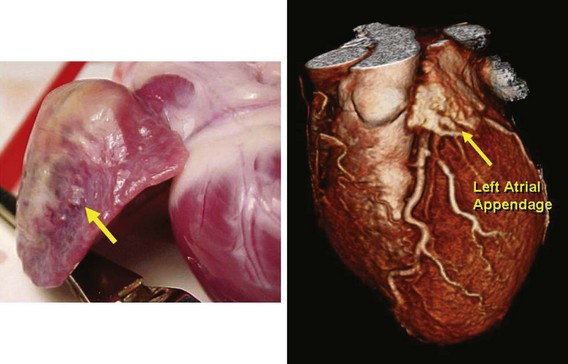
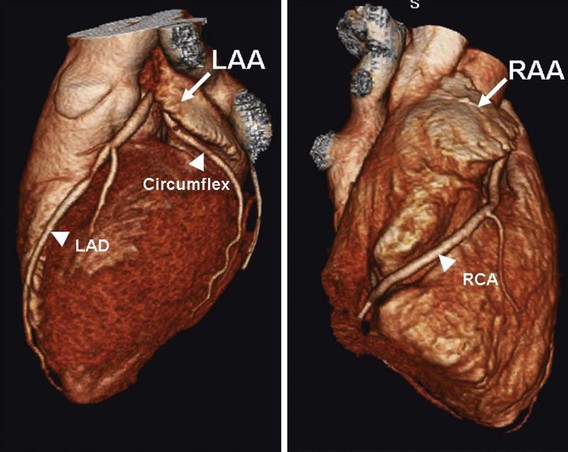
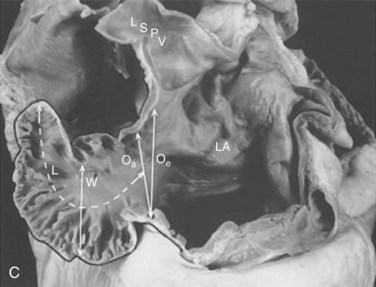
The LAA is a small epicardial chamber (see Fig. 47-1). It sits directly over the origins of the left main, left anterior descending (LAD), and left circumflex (LCx) coronary arteries. The right atrial appendage (RAA) is more tubular in shape, is longer, and generally covers the origin of the right coronary artery (Figs. 47-2 and 47-3). Hara reviewed the embryology, structure, and function of the LAA.35 The LAA originates from primordial atrial tissue, and the primordial left atrial wall becomes the tubular LAA. It is attached to the left atrial chamber, which suggests that the LAA may be an independent structure and perhaps not simply an appendage of the atrial wall. The rough, trabeculated cavitary surface of the LAA makes it a potential site for thrombus formation (Fig. 47-4).35 Plastic casts of the human LAA show substantial variations of anatomic shape and volume.36 Shape analysis shows that the LAA’s principal axis is angulated less than 100 degrees in about 56% of adults, and the remaining 46% are more severely bent. The volume of the LAA ranges from 770 to 19,270 mm3. Fifty-four percent of patients have two lobes, and the numbers range from one to four lobes (Figs. 47-5 and 47-6). There is no apparent relation to age or gender for any of these features of the LAA.