Figure 11.1 Types of hiatal hernia. The various types of hiatal hernia (Types I to IV) are depicted. (A) Sliding hiatal hernia. (B) True paraesophageal hernia with herniation of only a portion of the fundus of the stomach. (C) Paraesophageal hernia with herniation of the gastroesophageal junction and a portion of the fundus of the stomach. (D) Paraesophageal hernia with herniation of the gastroesophageal junction, fundus of the stomach, and portions of one or more other abdominal organs. (From: Sun R. Imaging for Surgical Disease. Philadelphia, PA: Lippincott Williams & Wilkins, 2014.)
 INDICATIONS/CONTRAINDICATIONS
INDICATIONS/CONTRAINDICATIONS
Repair is recommended for all symptomatic patients. The management of asymptomatic hernia remains the subject of debate, however, and warrants further discussion. The incidence of a truly asymptomatic giant PEH is uncommon in our experience, and more commonly, significant symptoms exist, but they have occurred so insidiously and have been present so long, that patients have learned to live with these significantly troublesome symptoms. Some studies have estimated that the risk of life-threatening complications from a PEH is lower than the risk of undergoing repair.6,7 When analyzing the findings of these studies, however, it is important to make note of the definition of minimally symptomatic or asymptomatic used; in the paper by Stylopoulos, minimal symptoms were defined as “heartburn that did not affect patient quality of life.” In our experience, the vast majority of patients with radiographic findings of large PEH will have obstructive symptoms, including dysphagia, postprandial bloating, and chest pain. On occasion, elderly patients in our clinics may deny difficulty swallowing and other symptoms, but will report significant and unintentional weight loss over the previous 5 to 10 years and, when questioned further, report substantial changes to their diet to avoid hard, and sometimes even soft solids, because the “food would not go down.”
When these hernias progress to requiring semi-urgent, nonelective repair, we and other surgeons have found that they are associated with a significantly increased risk of perioperative morbidity and mortality. In our series of 662 patients who underwent laparoscopic repair of giant PEH, patients admitted electively for laparoscopic repair had a postoperative mortality rate of 0.5% compared with 7.5% for patients who underwent urgent repair.5 This can be markedly higher when patients present with gastric necrosis, massive hemorrhage or severe aspiration pneumonia, albeit these more life threatening situations are less common. Thus, when evaluating patients who may be minimally symptomatic it is important to keep this data in mind. The risk of perioperative mortality and/or morbidity with elective and nonelective operation can be estimated to some degree by the size of the PEH, the patient’s functional status, the presence of comorbid conditions, and the patient’s symptom complex. We have recently shown that in patients with age-adjusted Charlson Comorbidity Index (CCI) scores of 5 or less, perioperative morbidity and mortality with elective laparoscopic repair is low and increases dramatically when performed urgently. We also showed that patients with very large PEH were much more likely to have obstructive symptoms, and to present urgently when compared with patients with smaller (<75% gastric herniation) PEH.8 Many of these urgent presentations occurred in patients who were over 80 years of age and in whom the presence of the PEH was known at an earlier age, even decades earlier, and not repaired. As such, we recommend elective surgical repair for most patients who have minimal symptoms and very large PEH because of the higher risk of mortality or complications after emergency surgery.
Relative contraindications to laparoscopic PEH repair include conditions that might preclude or increase the risk of all laparoscopic surgery, such as portal hypertension, dense abdominal adhesions preventing progress in the case, and significant hematologic clotting disorders, and contraindications to any surgery, such as inadequate cardiovascular function or the inability to tolerate general anesthesia. Age greater than 80 years is not a contraindication for laparoscopic PEH repair if these relative contraindications are manageable. Obesity is not a contraindication, but in the appropriate patient, a hernia repair along with a Roux-en-Y near-esophagojejunostomy may be a better option, especially in patients with comorbidities of obesity and a very high body mass index (BMI).9 It has also been noted that the risk for recurrent herniation with PEH repair in the morbidly obese patient may be increased5 and might be lowered by combining a hernia repair with a Roux-en-Y.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
Careful preoperative evaluation is essential. Careful symptom history includes: assessment of typical symptoms of gastroesophageal reflux disease (GERD) (heartburn, regurgitation), obstructive symptoms (dysphagia), chest or epigastric pain, postprandial pain, postprandial vomiting, and atypical symptoms (recurrent aspiration with or without associated pneumonia, cough, shortness of breath, and dyspnea on exertion). In patients with gastric volvulus in association with a PEH, which may be exacerbated in patients with a narrow crural opening, overt or occult bleeding can occur due to compromised blood supply to the herniated portion of the stomach. Even when overt strangulation or volvulus is not apparent, some patients with large PEH will experience varying degrees of gastritis. In some cases, ulceration and bleeding, occurs and patients present with hematemesis or melena but more often the presentation of bleeding is with a chronic anemia. In our experience, iron-deficiency anemia was diagnosed in a number of patients prior to surgical referral, but the relationship to the PEH went unrecognized; this association, when overlooked, can result in multiple blood transfusions, often over many years, before the patient is finally referred for surgical PEH repair. Following repair, the anemia resolves in the majority of patients.10,11 As such, assessment in patients with radiographic findings of PEH must include assessment for bleeding or chronic iron-deficiency anemia.
Additional preoperative evaluation includes the following.
 Blood work. Hemoglobin to assess anemia; serum albumin to evaluate nutritional status.
Blood work. Hemoglobin to assess anemia; serum albumin to evaluate nutritional status.
 Radiographic evaluation. Prior to operative intervention, all patients have radiographic evaluation of the PEH. The most common study is a barium esophagram, and this is currently standard for all of our elective cases unless the patient is unable to participate in the study. Computed tomography provides complementary information to the barium esophagram, such as identification of Type IV PEH, and can be used as a substitute for barium esophagram in urgent situations or for those patients unable to tolerate the barium esophagram. The barium esophagram and computed tomography scan provide an assessment of the location of the gastroesophageal junction, esophageal length, the amount of stomach herniated into the chest, if other organs may also be herniated, and whether a volvulus of the stomach is present. The barium esophagram may suggest that esophageal shortening is present, although, the absolute finding of a shortened esophagus can only be made at the time of surgery when the gastroesophageal junction cannot be delivered tension free into a subdiaphragmatic location. In addition, the barium esophagram can provide information about abnormal esophageal motility and associated abnormalities such as esophageal lesions, strictures or diverticula. A preoperative chest radiograph is obtained in all patients as well to identify other pulmonary pathology that might be present and assess for signs of either chronic lung injury or acute pneumonia secondary to aspiration.
Radiographic evaluation. Prior to operative intervention, all patients have radiographic evaluation of the PEH. The most common study is a barium esophagram, and this is currently standard for all of our elective cases unless the patient is unable to participate in the study. Computed tomography provides complementary information to the barium esophagram, such as identification of Type IV PEH, and can be used as a substitute for barium esophagram in urgent situations or for those patients unable to tolerate the barium esophagram. The barium esophagram and computed tomography scan provide an assessment of the location of the gastroesophageal junction, esophageal length, the amount of stomach herniated into the chest, if other organs may also be herniated, and whether a volvulus of the stomach is present. The barium esophagram may suggest that esophageal shortening is present, although, the absolute finding of a shortened esophagus can only be made at the time of surgery when the gastroesophageal junction cannot be delivered tension free into a subdiaphragmatic location. In addition, the barium esophagram can provide information about abnormal esophageal motility and associated abnormalities such as esophageal lesions, strictures or diverticula. A preoperative chest radiograph is obtained in all patients as well to identify other pulmonary pathology that might be present and assess for signs of either chronic lung injury or acute pneumonia secondary to aspiration.
 Flexible endoscopy. Preoperative or intraoperative endoscopy is always performed by the operating surgeon to evaluate gastric and esophageal viability, identify associated abnormalities, such as Barrett’s esophagus or esophageal malignancy, identify the location of the gastroesophageal junction, and assess and estimate esophageal length, which may have been difficult to evaluate due to the anatomic distortion created by the PEH. It is critical for the surgeon to perform their own endoscopy and not rely on the findings on endoscopy reported by others as the anatomy of the esophagus and stomach are often distorted and can be difficult to evaluate when unaccustomed to evaluation of PEH.
Flexible endoscopy. Preoperative or intraoperative endoscopy is always performed by the operating surgeon to evaluate gastric and esophageal viability, identify associated abnormalities, such as Barrett’s esophagus or esophageal malignancy, identify the location of the gastroesophageal junction, and assess and estimate esophageal length, which may have been difficult to evaluate due to the anatomic distortion created by the PEH. It is critical for the surgeon to perform their own endoscopy and not rely on the findings on endoscopy reported by others as the anatomy of the esophagus and stomach are often distorted and can be difficult to evaluate when unaccustomed to evaluation of PEH.
 Pulmonary function testing. We do not routinely obtain pulmonary function testing (PFT) for elective repair of a large PEH; however, when shortness of breath or dyspnea on exertion is present, PFTs may offer important information and risk assessment. This can be due to the space-occupying effects of the gastric herniation into the posterior mediastinum, with local effects on both the heart and the adjacent lung, but may also be due to chronic aspiration and in some cases repeated pneumonias. In cases of complete intrathoracic stomach, the herniation may occupy as much as 40% to 50% of the volume of the right or left hemithorax. In these extremes, PFTs may be useful for assessing the degree of pulmonary impairment but it may be difficult to determine how much of this is due to the hernia versus coexisting lung disease.
Pulmonary function testing. We do not routinely obtain pulmonary function testing (PFT) for elective repair of a large PEH; however, when shortness of breath or dyspnea on exertion is present, PFTs may offer important information and risk assessment. This can be due to the space-occupying effects of the gastric herniation into the posterior mediastinum, with local effects on both the heart and the adjacent lung, but may also be due to chronic aspiration and in some cases repeated pneumonias. In cases of complete intrathoracic stomach, the herniation may occupy as much as 40% to 50% of the volume of the right or left hemithorax. In these extremes, PFTs may be useful for assessing the degree of pulmonary impairment but it may be difficult to determine how much of this is due to the hernia versus coexisting lung disease.
 Esophageal physiology testing. For large PEH, particularly in patients with primarily obstructive symptoms, pH studies are not routinely performed because the primary indication for repair is related to the mechanical obstruction of the esophagus and stomach rather than sphincter incompetence and reflux disease. A negative pH study would not change the need for operative repair. Manometry can be useful in some patients in whom an esophageal motility disorder is suspected, but should be undertaken with caution as the placement of the catheter can result in perforation of the esophagus or stomach when the anatomic derangements due to the herniation are not recognized. However, if simple manometric assessment of the esophageal body and motor function are being performed, this is generally well tolerated and may help in determining the type of fundoplication used during repair of the PEH.
Esophageal physiology testing. For large PEH, particularly in patients with primarily obstructive symptoms, pH studies are not routinely performed because the primary indication for repair is related to the mechanical obstruction of the esophagus and stomach rather than sphincter incompetence and reflux disease. A negative pH study would not change the need for operative repair. Manometry can be useful in some patients in whom an esophageal motility disorder is suspected, but should be undertaken with caution as the placement of the catheter can result in perforation of the esophagus or stomach when the anatomic derangements due to the herniation are not recognized. However, if simple manometric assessment of the esophageal body and motor function are being performed, this is generally well tolerated and may help in determining the type of fundoplication used during repair of the PEH.
 SURGERY
SURGERY
It is important that in the preoperative setting a thorough discussion of the risks and benefits of the operation, including the risk for perioperative death or other adverse outcomes, recurrent hernia, and potential need for reoperation is undertaken. After repair, patients are followed in our clinics long term to monitor for recurrent symptoms or hernia. This close attention to long-term outcomes facilitates early recognition of recurrent symptoms, including dysphagia, and appropriate interventions to assist with patient comfort and satisfaction with quality of life. It is important for the surgeon to remain engaged in this process as the patient’s primary care physicians and even their gastroenterologists may incorrectly attribute symptoms to the PEH repair or fail to recognize correctible problems that are related to the PEH repair.
Once the patient agrees to proceed, final preparations for surgery are made. All patients have a preoperative electrocardiogram, chest radiograph, type and screen and received modified bowel prep with 1 L of polyethylene glycol electrolyte solution. We have found this to be helpful, especially in elderly patients or in patients prone to constipation. Any patients with risk factors for coronary heart disease, including age, hypertension, history of smoking or prior history of coronary disease also undergo cardiac evaluation with a minimum of an exercise or persantine-thallium stress test to determine whether significant coronary disease is present. If the stress test is positive, cardiology consultation is obtained prior to operative intervention, except in emergency cases.
On the day of surgery, patients receive 5,000 units of heparin subcutaneously prior to induction of anesthesia, preferably in the preoperative holding area.12 In the operating room, general endotracheal anesthesia is induced and flexible endoscopy performed by the surgeon. Care is taken to minimize air insufflation during the endoscopic evaluation. The esophagus is inspected and the stomach is decompressed as much as possible, given the anatomy of the patient. The patient is then positioned for laparoscopy. Our preferred approach for positioning of the patient is supine with the surgeon on the patient’s right side and the assistant on the left. A subhepatic liver retractor is used, so the patient is placed to the far right of the operating room table. A foot stop is placed to facilitate reverse Trendelenburg positioning. Sequential compression devices are placed on the legs bilaterally. A Foley catheter is placed. The patient’s arms are rotated away from the patient, secured to an arm board at a 45-degree angle from the bed and carefully padded. This angle provides adequate access to the operating table and minimizes the risk of stretch injury to the brachial plexus. The abdomen is then prepped and draped and intravenous antibiotics administered for wound infection prophylaxis.
Proper port placement is the key to successful execution of the operation. Because of the extensive mediastinal dissection required to reduce the hernia sac and to fully mobilize the esophagus, placement of the ports in the upper aspect of the abdomen is critical. To accomplish this, we identify the midline from the xiphoid to the umbilicus and use a skin marker to divide the distance into thirds (Fig. 11.2). In morbidly obese patients, attention to the distance from the costal margins to the pelvis using the bony anatomy will assist with gauging proper port placement. In the majority of patients, five ports are used. Using the open, blunt port cut down technique, a 10-mm Hassan port,13 is placed in the right paramedian line approximately one-third of the way from the xiphoid to the umbilicus, taking care to avoid dissection into the falciform ligament. Insufflation pressures are set at between 12 and 15 mm Hg, depending on the patient’s hemodynamics and intraoperative visibility. In compromised patients with poor cardiopulmonary risk, we have found that many can be repaired with insufflation pressure ranging from 8 to 10 mm Hg routinely. Once we have confirmed proper positioning of the Hassan port within the peritoneal cavity, full insufflation is achieved. Port placement then proceeds under direct vision. The assistant’s ports are positioned to the left of the midline. The assistant’s left hand holds the camera, which is passed via a 5- or 10-mm port in the left paramedian line at approximately the same level or slightly lower than the Hassan port in the right paramedian line. The assistant’s right hand is directly below the costal margin in the midclavicular line and is used for retraction. The surgeon’s ports include the Hassan port, through which an energy device is passed for use in sharp dissection. A 5-mm port for the surgeon’s left hand is placed in the right midclavicular line directly below the costal margin. It is critical that the subcostal ports on either side be at least a hands breath (9 to 10 cm) from the paramedian ports as closer positioning creates the potential for interference between the instruments during the procedure. Liver retraction can be accomplished either using a subxiphoid position or through a 5-mm port in the far right lateral subcostal position, depending upon the type of liver retractor to be used.
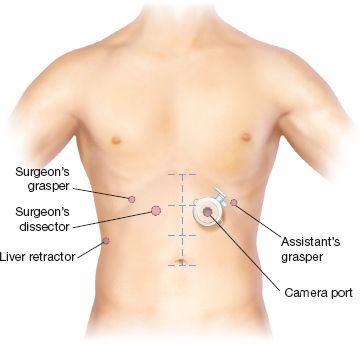
Figure 11.2 Surgeon and port position. Port placement and instrument positions are shown. In a nonobese patient, the ports are positioned one-third of the distance from the xiphoid to the umbilicus. In obese patients, this measure is often inaccurate because of the increased abdominal circumference. In this situation, the patient’s bony anatomy can be used to determine appropriate placement with an imaginary line across the abdomen at top of the anterior superior iliac spines serving as a marker for the normal distance to the umbilicus.



