Evidence from multiple large prospective studies suggests that a common polymorphism that encodes an arginine (Arg)–to–tryptophan substitution at position 719 in the KIF6 gene is associated with coronary heart disease (CHD) and reduction in coronary events from statin therapy. Carriers of the 719Arg allele were at greater risk for primary and secondary CHD events, and statin therapy significantly reduced coronary events in 719Arg carriers but not in noncarriers. The number needed to treat to prevent a single CHD event ranged from 10 to 20 for 719Arg carriers, compared to >80 for noncarriers in the Cholesterol and Recurrent Events (CARE) study, the West of Scotland Coronary Prevention Study (WOSCOPS), the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER), and the Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis In Myocardial Infarction 22 (PROVE IT–TIMI22) study. In conclusion, assessment of 719Arg carrier status holds promise for stratification of coronary event risk and for selection of optimal therapy in primary and secondary CHD prevention.
Statins reduce coronary heart disease (CHD) events by as much as 37% in clinical trials by lowering low-density lipoprotein (LDL) cholesterol levels and by other (pleiotropic) effects on inflammation, thrombogenesis, and arterial vasomotor function. However, response to statin therapy varies among patients in part because of genetic factors. For example, a study in the Treating to New Targets (TNT) cohort (5,745 subjects) has provided strong evidence (p = 6 × 10 −30 ) that statin therapy lowered LDL cholesterol more in carriers of the apolipoprotein E ε2 variant than in carriers of the ε3 variant, while ε4 variant carriers experienced less LDL cholesterol lowering. Recently, a common polymorphism (rs20455) that encodes an arginine (Arg)–to–tryptophan (Trp) substitution at position 719 (Trp719Arg) of KIF6, a member of the kinesin 9 family, has been shown to predict risk for CHD and reduction in coronary events by statin therapy. Here, we review the data supporting the association between the KIF6 polymorphism and CHD and the association between the KIF6 polymorphism and event reduction by statin therapy. We also discuss the putative biologic role of this kinesin in the cause of CHD and the potential use of KIF6 genotyping in the prevention and management of CHD.
KIF6 Polymorphism and Risk for Coronary Events
Carriers of the KIF6 719Arg allele (Arg/Arg homozygotes and Arg/Trp heterozygotes) have increased risk for CHD in multiple large prospective cohort studies, compared to noncarriers of the 719Arg allele (Trp/Trp homozygotes). The first results from a prospective study were obtained when the association of the KIF6 polymorphism with CHD was investigated in the placebo groups of the Cholesterol and Recurrent Events (CARE) trial and the West of Scotland Coronary Prevention Study (WOSCOPS). In these studies, only carriers of the KIF6 719Arg allele had increased risk for CHD (p <0.05 after Bonferroni’s correction for the testing of 35 single-nucleotide polymorphisms that had all been previously reported to be associated with CHD; Figure 1 ). Carriers of the 719Arg allele were also at increased risk for CHD in patients with previous vascular disease in the placebo group of the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) and in the placebo group of an initial analysis of 20% of the Heart Protection Study (HPS). In addition, the KIF6 719Arg allele was associated with increased risk for CHD in multiple population-based observational studies of cardiovascular diseases, including the Atherosclerosis Risk in Communities (ARIC) study, the Cardiovascular Health Study (CHS), and the Women’s Health Study (WHS). These studies encompass a broad spectrum of populations. For example, 85% of CARE patients and all WOSCOPS patients were men, and all WHS participants were women; WOSCOPS patients and ARIC subjects were middle aged, whereas CHS participants and PROSPER patients were elderly; subjects in CARE were secondary CHD prevention patients, whereas those in the WHS were initially healthy, primary CHD prevention subjects. Thus, the repeated association of the KIF6 polymorphism with CHD in these studies suggests that this polymorphism is likely to predict risk in the broad population.
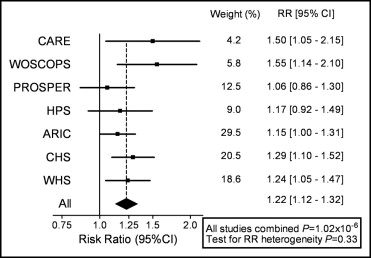
The magnitude of increased risk in KIF6 719Arg carriers, compared to noncarriers, ranged from 1.06 in the placebo arm of PROSPER to 1.55 in the placebo arm of WOSCOPS. In a meta-analysis of 7 prospective studies that comprised 3 prospective studies of those without CHD events at baseline and the placebo groups of 4 statin trials, the risk ratio was 1.22 (95% confidence interval 1.12 to 1.32; combined p = 1.02×10 −6 ; Figure 1 ). The risk estimate for 719Arg carriers in individual cohorts was not appreciably altered after adjustment for traditional risk factors, including age, blood pressure, diabetes, smoking, LDL cholesterol or high-density lipoprotein cholesterol, suggesting that the KIF6 polymorphism is a new, independent risk factor for CHD.
The prevalence of KIF6 719Arg carriers is about 59% among Caucasians and varies among other ethnic groups ( Table 1 ). Preliminary results suggest that the 719Arg allele is also associated with increased risk for CHD in other ethnic groups. For example, African American carriers of the 719Arg allele had increased risk for myocardial infarction in the CHS cohort (odds ratio 4.14, 95% confidence interval 0.57 to 29.9) and of CHD in the ARIC cohort (hazard ratio 1.3 per 719Arg allele carried); these observations, however, require replication in large, independent samples.
| 719Arg Carriers | 719Arg Noncarriers | |||
|---|---|---|---|---|
| Population | Arg/Arg + Arg/Trp | Arg/Arg | Arg/Trp | Trp/Trp |
| African: Yoruba | 99% | 82% | 17% | 1% |
| African American | 94% | 61% | 33% | 6% |
| Asian: Chinese and Japanese | 72% | 25% | 47% | 28% |
| Caucasian: ARIC participants | 59% | 13% | 46% | 41% |
| Hispanic: Costa Rican | 58% | 13% | 45% | 42% |
⁎ Based on the HapMap data set ( http://www.hapmap.org ) and Celera (Alameda, California) data.
An association between the KIF6 Trp719Arg polymorphism and CHD was not observed in the Wellcome Trust Case Control Consortium. However, the association was not analyzed according to statin use in that study, and the inclusion of subjects receiving statin therapy might have reduced the power of the case-control design because risk conferred by the 719Arg allele may be ameliorated by statin therapy (see the next section). The Ottawa Heart Genomic Study also investigated the KIF6 Trp719Arg polymorphism. However, cases in this study were defined as patients with >50% coronary narrowing. This definition is different from the coronary event end points used in the studies that did find an association between the KIF6 Trp719Arg polymorphism and CHD. The difference in the end points that were tested could explain the lack of association.
KIF6 Polymorphism and Coronary Events in Randomized Studies of Statin Therapy
Carriers of the KIF6 719Arg allele have been associated with coronary event reduction from statin therapy in the retrospective analyses of several large clinical trials. In the CARE and WOSCOPS studies, pravastatin therapy significantly reduced coronary events in carriers of the 719Arg allele but not in noncarriers. For instance, in WOSCOPS, 719Arg carriers had a 50% relative risk reduction of coronary events during pravastatin therapy, compared to a nonsignificant reduction of 9% in noncarriers. In the original PROSPER study, only patients with previous vascular disease received significant benefit from pravastatin therapy, and within this group, only 719Arg carriers, but not noncarriers, received a significant reduction of coronary events during pravastatin therapy. In the Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis In Myocardial Infarction 22 (PROVE IT–TIMI22) study, high-dose (80 mg/day) atorvastatin, compared to standard dose pravastatin, also resulted in a reduction of the number of deaths or major cardiovascular events in 719Arg carriers, but not in noncarriers ( Figure 2 ). A summary of the absolute risk reduction according to 719Arg carrier status is shown in Figure 3 . The absolute risk reduction in the WOSCOPS primary prevention trial was 5.5% in carriers compared to 0.1% in noncarriers ( Figure 3 ). The absolute risk reduction in the secondary prevention trials (CARE, PROSPER, and PROVE IT–TIMI 22) ranged from 5% to 10% in 719Arg carriers compared to 0.4% to 1.2% in noncarriers.
In patients with acute coronary syndromes (PROVE IT–TIMI 22), the reduction of deaths or major vascular events from high-dose statin therapy reached significance at 30 days in 719Arg carriers, whereas in noncarriers, event reduction was not significant at any time point ( Figure 4 ). The restriction of early efficacy to 719Arg carriers did not appear to have been mediated through LDL cholesterol lowering, because on-therapy LDL cholesterol did not differ between 719Arg carriers and noncarriers in either the PROVE IT–TIMI 22 study or the PROSPER study. Furthermore, in PROVE IT–TIMI 22, C-reactive protein levels did not differ between carriers and noncarriers. Taken together, these results suggest that the KIF6 polymorphism is a predictor of coronary event reduction during statin therapy and that at least part of the event reduction from statin therapy may be mediated by mechanisms that are independent of lowering of the blood level of LDL cholesterol.




