Kawasaki Disease (Mucocutaneous Lymph Node Syndrome)
Sarah D. de Ferranti
Jane C. Burns
Jane W. Newburger
Kawasaki disease (KD), first described in Japan in 1967 by Dr. Kawasaki, is now encountered worldwide (1,2). The first US cases were reported from Hawaii in 1976 by Melish et al. (3). Despite intensive research, the cause of KD remains unknown. The principal symptoms and associated features of the acute phase of the syndrome are shown in Tables 58.1 and 58.2 (4,5). Although the cardiovascular manifestations of KD were not appreciated originally, by the mid-1970s, researchers reported that about 2% of affected children died suddenly in the subacute or convalescent stage of this illness due to myocardial infarction caused by acute thrombosis within coronary artery aneurysms or rarely from aneurysm rupture (6). In a recent study in Japan that followed 6,576 patients who had had KD between July 1982 and December 1992, with survival data obtained until December 2009, standardized mortality ratios (SMRs) based upon Japanese vital statistics data showed that survival among children without coronary aneurysms was similar to that of the general population. However, among those with cardiac sequelae, SMR was 1.86 (95% CI: 1.02 to 3.13) (7).
TABLE 58.1 Principal Symptoms in Kawasaki Disease | |||
|---|---|---|---|
|
Epidemiologic Features
KD is now one of the leading causes of acquired cardiovascular disease in the young (rheumatic fever being the other-see Chapters 59 and 80) and appears to be increasing in incidence. In Japan, nationwide biennial active surveillance shows the incidence of KD in children under 5 years of age has gradually increased from 74 per 100,000 in 1987 to 140 per 100,000 in 2000, and to 239 per 100,000 in 2009–2010 (8,9,10). In children younger than 5 years of age, US estimates based on 2006 hospital discharge data suggest the annual incidence is 20 per 100,000 children (11). The typical KD patient is more likely to be male (ratio of males to females 1.5:1) and <5 years of age (80%). In US children under 5 years of age, the prevalence of KD is the highest in Asians and Pacific Islanders (32.5 per 100,000), intermediate in non-Hispanic Blacks (16.9 per 100,000) and Hispanics (11.1 per 100,000), and lowest in Whites (9.1 per 100,000) (11).
Coronary aneurysms are the most important complication of KD. Young infants have the highest rate of coronary artery aneurysm formation and often present with incomplete clinical criteria (12,13). Children older than age 8 also have a higher rate of coronary involvement (14,15). The US rates of coronary artery aneurysm according to race/ethnicity have been estimated using administrative data; rates were highest in Hispanics (5.9%), followed by white non-Hispanics (3.4%), with blacks having lower rates (1.8%) (16); the study design did not allow the authors to determine whether differences in rates of aneurysms among racial/ethnic groups were
related to late or inadequate treatment versus higher relative risk. A retrospective review of outcomes in KD children treated with intravenous immunoglobulin (IVIG) at major academic centers in Japan and the United States used coronary artery z-scores (internal diameter normalized for body surface area [BSA]) (17). Of 568 US patients, 24.3% had a z-score for the right coronary artery (RCA) or left anterior descending coronary artery (LAD) between 2.5 and 5.0 and 5.8% had a z-score >5.0. This included 62 patients (11.5%) who were treated beyond 10 days after onset of fever.
related to late or inadequate treatment versus higher relative risk. A retrospective review of outcomes in KD children treated with intravenous immunoglobulin (IVIG) at major academic centers in Japan and the United States used coronary artery z-scores (internal diameter normalized for body surface area [BSA]) (17). Of 568 US patients, 24.3% had a z-score for the right coronary artery (RCA) or left anterior descending coronary artery (LAD) between 2.5 and 5.0 and 5.8% had a z-score >5.0. This included 62 patients (11.5%) who were treated beyond 10 days after onset of fever.
TABLE 58.2 Features Associated with Acute Kawasaki Disease | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Recurrence rates vary by ethnic group, with the highest recurrence rate of 3.6% reported from Japan (8). In a population of largely European descent in Ontario, Canada, the rate of recurrence of KD was 2.9 episodes/1,000 patient-years (18). Based on the 2009–2010 survey in Japan, 1.6% of KD patients had at least one affected sibling and 0.7% had at least one parent with a history of KD (8). These observations support the role of genetic factors in susceptibility to KD.
Etiology and Pathogenesis
An etiologic agent for KD has not been identified, despite extensive research into infectious, immunologic, and genetic causes. An infectious trigger is suggested by the epidemiologic characteristics of this syndrome, especially its tendency to target young children, clustering of cases in time and space, and a predilection for winter and spring months (19,20). Person-to-person spread has not been documented. Search for a novel virus has been spurred on by the finding of intracytoplasmic inclusion bodies in bronchial epithelial cells (21,22). An association of tropospheric wind patterns with peaks in KD cases in Japan, Hawaii, and the West Coast has focused an investigation on aerosol particles carried by these winds (23). Suffice it to say that the environmental trigger for KD remains unknown.
Because the winter–spring season is also a time when respiratory viruses abound, it is common for children with KD to have concomitant infection with common respiratory viruses (24,25). Among these, human adenovirus is the most likely to enter the differential diagnosis of KD because of the presence of prolonged fever, elevated white blood cell count, and erythrocyte sedimentation rate (ESR). Studies on gene expression profiling have suggested a very different signature for adenovirus compared to KD, but adenovirus PCR can be positive because of reactivation of latent infection with viral shedding (26,27). The clinical features of KD are similar to those of toxin-related diseases, such as scarlet fever or toxic shock syndrome (28). Furthermore, other processes can mimic the presentation of KD, such as drug reactions (Table 58.3).
The immune response in acute KD patients has been an active area of investigation but most of the work to date remains descriptive. The most widely accepted paradigm is that an environmental trigger sparks a detrimental immune response in a host who is genetically or otherwise vulnerable. The activation of both the innate and adaptive immune systems, and the infiltration of cytotoxic CD8+ T-cells and IgA-secreting plasma cells into the arterial wall during the acute phase of illness have all been described (29,30,31,32). Proinflammatory and anti-inflammatory cytokines are all upregulated during the acute illness, including interleukin (IL)-1, IL-2, IL-6, IL-10, and tumor necrosis factor alpha (TNF-α) (33). Proinflammatory cytokines appear to render the vascular endothelium susceptible to lysis by antibodies (34). Activated vascular endothelium expresses inflammatory antigens such as selectins and adhesion molecules (35). Gene expression studies have documented high levels of transcripts from genes in the IL-1 signaling pathway (36). However, to date, there is no “diagnostic signature” of inflammatory markers that permits the reliable distinction of KD from other inflammatory processes. The role of T-cell regulation is emerging as an important theme in disease pathogenesis, response to IVIG, and the self-limited nature of the disease (37).
A complex genetic predisposition of the host appears to be important in the pathogenesis of KD. Both genome-wide association studies and family linkage studies have explored the association of single nucleotide polymorphisms to susceptibility to KD or to the development of aneurysms (38,39), and more recently DNA methylation patterns have also been explored as risks for KD (40). Genetic variation in genes in the calcium signaling pathway and Fc gamma receptors has emerged as important influence on KD susceptibility (41,42,43). Response to IVIG has been further linked to polymorphisms in the inhibitory Fc gamma receptor (44). Susceptibility to coronary artery aneurysms has been linked to genetic variation in genes in the transforming growth factor beta (TGFβ) pathway (45). The influence of subtle variation in these different pathways serves as a signpost declaring the importance of these pathways in disease pathogenesis and has thus led to new clinical trials. Cyclosporine, which specifically targets the calcium signaling pathway, and atorvastatin, which targets effects of TGFβ signaling, are both being tested in KD patients (46,47).
TABLE 58.3 Diseases and Disorders with Clinical Findings Similar to Kawasaki Disease | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
In summary, after more than four decades of research, investigators are still searching for the cause(s) of this mysterious childhood illness. It is likely that genetic predisposition affects both disease susceptibility and the development of coronary artery aneurysms. Progress in genetics and genomics may further define the population at risk, while advances in proteomics, metabolomics, and gene expression profiling may yield additional insights into pathogenesis and improved methods of molecular diagnosis.
Pathology
Our understanding of the stages of cardiovascular pathology is based on the analysis of available postmortem specimens and endomyocardial biopsies (48,49) (Table 58.4). Three vasculopathic processes have been delineated including (1) an acute, self-limited necrotizing arteritis with neutrophil infiltration, (2) a subacute vasculitis that is predominantly T-cell mediated, and (3) luminal myofibroblastic proliferation with the transition of medial and adventitial smooth muscle cells into myofibroblasts that then proliferate and result in stenosis of the lumen (Fig. 58.1). The destructive changes that lead to aneurysm formation are most common in the proximal segments and branching points of the coronary arteries, suggesting a role for hemodynamic stress in the development of aneurysms. In addition to the dramatic
changes in the arterial wall, histologic evidence of myocarditis is universally present in the acute phase. Endomyocardial biopsies show mononuclear cell infiltration and edema within the myocardium (50,51). Valvulitis may affect the mitral and aortic valves. Noncoronary arteries, such as iliac, femoral, axillary, and renal, can also be affected, although much less frequently and only in patients who have coronary aneurysms. Involvement of intracranial arteries or intraparenchymal vessels within abdominal organs is extremely rare.
changes in the arterial wall, histologic evidence of myocarditis is universally present in the acute phase. Endomyocardial biopsies show mononuclear cell infiltration and edema within the myocardium (50,51). Valvulitis may affect the mitral and aortic valves. Noncoronary arteries, such as iliac, femoral, axillary, and renal, can also be affected, although much less frequently and only in patients who have coronary aneurysms. Involvement of intracranial arteries or intraparenchymal vessels within abdominal organs is extremely rare.
TABLE 58.4 Stages of Cardiovascular Pathology in Kawasaki Disease | |||||
|---|---|---|---|---|---|
|
Clinical Features: Systemic
The acute phase of KD often is preceded by prodromal upper respiratory or gastrointestinal symptoms (52). The beginning of KD is marked by an abrupt onset of high fever, accompanied by rash, bilateral nonexudative conjunctival injection, reddened and cracked lips, erythema of the buccal mucosa, strawberry tongue, nonsuppurative cervical lymphadenitis classically with a single large node (≥1.5 cm), and erythema and edema of the hands and feet (1). The rash may take different forms, but bullae and vesicles are rare. The rash frequently begins in the diaper area and spreads to the torso and extremities. It may be evanescent, especially in young infants (Table 58.1). Erythema and edema of the hands and feet may be accompanied by fusiform swelling of the proximal interphalangeal joints of the hands. Patients may refuse to move their hands or bear weight on their feet. Occasionally, they may show transient Raynaud phenomenon and inflammation at the site of previous Bacille Calmette–Guérin (BCG) vaccinations has been reported in as many at 50% of patients in areas where BCG vaccinations are commonly administered (53,54). The most dramatic extremity symptom is gangrene of fingers and toes, which occurs rarely in very young infants, mostly of non-Asian background (55).
At least moderate elevation of acute phase reactants, that is, the ESR or C-reactive protein (CRP), is almost universal at the time of presentation. Both ESR and CRP should be measured because these test values may be discrepant at presentation (56), and because IVIG is associated with increases in the ESR while CRP is not affected by IVIG treatment. Elevations of liver function tests, including plasma gamma-glutamyl transpeptidase, transaminases, and bilirubin, are also common (28,57). Albumin synthesis declines in the acute phase, and hypoalbuminemia is common. Patients can have urethritis and phimosis (in uncircumcised males), sometimes accompanied by dysuria and sterile pyuria. Orchitis may occur in boys. Urinalysis shows the so-called sterile pyuria, with white cells (often in the range of 10 to 50/high-power field [HPF]) noted on microscopic evaluation but not by dipstick. Clinical and spinal fluid findings of aseptic meningitis may be present in the acute phase. Lumbar puncture may show findings compatible with aseptic meningitis, with a predominance of mononuclear cells, but with normal glucose and protein levels (58).
This acute phase is followed by a subacute phase, which occurs from the 2nd to the 4th week of illness. During this time, most patients experience desquamation starting in the subungual regions and spreading to the palms and soles. In addition to the principal symptoms, there may be hepatomegaly, hydrops of the gallbladder (59), transient jaundice, and abnormal liver function tests. Some patients develop transient diarrhea and abdominal discomfort. In some patients, arthralgia or arthritis appears late in the acute or subacute phase and very rarely may last up to 4 months (3). Transient and isolated peripheral nerve impairment such as facial palsy, phrenic nerve paralysis, or sensorineural hearing loss has also been described (60,61). Associated clinical and laboratory findings are summarized in Table 58.2.
If the patient remains untreated or is treated with aspirin only, the febrile course usually lasts from 1 to 3 weeks. Transient anemia
and leukocytosis with increased numbers of neutrophils and bands are common but significant anemia should prompt an investigation for immune-mediated hemolysis associated with IVIG (62,63). The platelet count increases in the 2nd and 3rd weeks of illness. Thrombocytosis and elevated sedimentation rate or CRP will gradually subside by the 6th to 8th week of illness.
and leukocytosis with increased numbers of neutrophils and bands are common but significant anemia should prompt an investigation for immune-mediated hemolysis associated with IVIG (62,63). The platelet count increases in the 2nd and 3rd weeks of illness. Thrombocytosis and elevated sedimentation rate or CRP will gradually subside by the 6th to 8th week of illness.
Recurrence and Recrudescence
Rarely, KD can recur in patients who have recovered completely from the original episode (reported as high as 3% in Japan). Children who have recurrent disease appear to be at increased risk of coronary complications (64). Recurrent KD must be distinguished from the so-called recrudescence, which occurs in the acute phase of illness characterized by temporary remission of fever and other symptoms followed by relapsing fever. In the current era, recrudescent fever most commonly occurs after an initial response to IVIG therapy. Children with recrudescent fever, similar to those with recurrent disease, are at higher risk of coronary artery complications. It is important to instruct the family to monitor the patient’s temperature daily after hospital discharge until the patient has been afebrile for a week, so that additional anti-inflammatory treatment can be instituted if fever recurs.
Diagnosis and Differential Diagnosis
The 2004 American Heart Association (AHA) epidemiologic case definition of KD requires the presence of ≥4 days of fever and at least four of the five principal clinical features, including bilateral nonexudative conjunctivitis, erythema of the lips and oral mucosa, changes in the extremities, rash, and cervical lymphadenopathy (Table 58.1) (4). When coronary artery disease is documented, the diagnosis of KD can be made with fewer than four principal features (incomplete KD). All clinical features are rarely present at the same time, so the diagnosis requires sequential evaluation of the patient with detailed day-by-day history of the present illness.
No specific diagnostic test exists for KD, and many illnesses mimic KD (Table 58.3) such as viral or rickettsial exanthems (measles, Epstein–Barr virus infection, and Rocky Mountain spotted fever), scarlet fever, leptospirosis, toxic shock syndrome, juvenile rheumatoid arthritis with or without macrophage activation syndrome (65), Stevens–Johnson syndrome, reaction to drugs, and hypersensitivity to mercury (28). Careful history, physical examination, and appropriate laboratory tests are necessary to exclude these conditions.
Although most cases fulfill the principal diagnostic criteria listed in Table 58.1, about 15% of cases have incomplete clinical presentations with coronary artery complications (66). In any child with unexplained fever lasting >5 to 7 days with some of the above-mentioned laboratory findings, the diagnosis of incomplete KD should be considered and echocardiography should be obtained (see Fig. 58.2). Very young infants are especially likely to present with incomplete KD; indeed, some have fever as their only manifestation. For this reason, echocardiography should be performed on any infant younger than age 6 months with fever duration of ≥7 days, elevation of CRP and/or ESR, and no other explanation for the febrile illness.
Algorithm for Clinical Evaluation and Treatment of Children with Suspected Kawasaki Disease
When the epidemiologic case definition for KD was first constructed by a committee of the Japanese Ministry of Health in 1970, the association of coronary artery sequelae with KD was not appreciated (67). Indeed, at that time, neither an effective treatment nor a noninvasive method of assessing coronary artery abnormalities was available. The original case definition was designed to be highly specific (i.e., to yield a low false-positive rate), but its sensitivity was limited and did not include echocardiographic data as part of the diagnostic criteria. Because coronary artery aneurysms are now well recognized to occur not only in children with typical KD but also in children with incomplete features of KD, or other febrile illnesses (68,69)
and because IVIG therapy must be given within the time frame of 7 to 10 days to be most effective in preventing coronary artery aneurysms, an algorithm for evaluation and treatment of the child with suspected KD was developed by members of the AHA Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease that incorporates echocardiographic findings and laboratory tests (Fig. 58.3) (4). This algorithm as originally published was not evidence based, but rather reflected the consensus of experts. Salient features of
patients with incomplete KD include laboratory tests indicating inflammation (e.g., elevated ESR, CRP, and white blood cell count), evidence of anemia, elevated alanine aminotransferase (ALT) and low albumin, and sterile pyuria. Ectasia and/or lack of tapering of the coronary arteries, left ventricular dysfunction, and, definitively, aneurysms support the diagnosis of KD in the incomplete presentation. The performance of the 2004 AHA recommendations for treatment of suspected or definite KD has been evaluated retrospectively in patients with definite coronary artery aneurysms (70). Of those with aneurysms, 97% would have received IVIG therapy with application of the classic criteria together with the algorithm for suspected incomplete KD (67).
and because IVIG therapy must be given within the time frame of 7 to 10 days to be most effective in preventing coronary artery aneurysms, an algorithm for evaluation and treatment of the child with suspected KD was developed by members of the AHA Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease that incorporates echocardiographic findings and laboratory tests (Fig. 58.3) (4). This algorithm as originally published was not evidence based, but rather reflected the consensus of experts. Salient features of
patients with incomplete KD include laboratory tests indicating inflammation (e.g., elevated ESR, CRP, and white blood cell count), evidence of anemia, elevated alanine aminotransferase (ALT) and low albumin, and sterile pyuria. Ectasia and/or lack of tapering of the coronary arteries, left ventricular dysfunction, and, definitively, aneurysms support the diagnosis of KD in the incomplete presentation. The performance of the 2004 AHA recommendations for treatment of suspected or definite KD has been evaluated retrospectively in patients with definite coronary artery aneurysms (70). Of those with aneurysms, 97% would have received IVIG therapy with application of the classic criteria together with the algorithm for suspected incomplete KD (67).
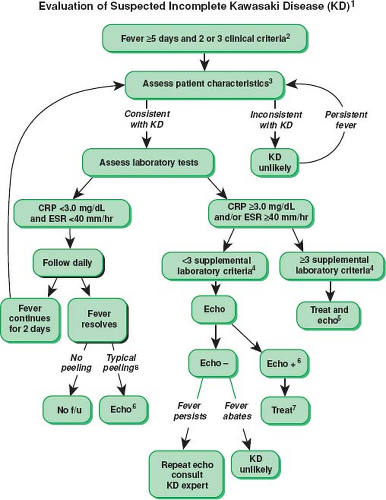 Figure 58.3 This algorithm is a guide to the evaluation of patients with suspected incomplete Kawasaki disease (KD). (1) In the absence of gold standard for diagnosis, this algorithm cannot be evidence based, but rather, represents the informed opinion of the expert committee. Consultation with an expert should be sought any time assistance is needed. (2) Infants ≤6 months old on day ≥7 of fever without other explanation should undergo laboratory testing, and if evidence of systemic inflammation is found, an echocardiogram should be obtained, even if the infants do not fulfill clinical criteria for KD. (3) Patient characteristics suggesting KD are listed in Table 58.2. Characteristics suggesting disease other than KD include exudative conjunctivitis, exudative pharyngitis, discrete intraoral lesions, bullous or vesicular rash, or generalized adenopathy. For these patients, one should consider alternative diagnoses (see Diagnosis and Differential Diagnosis; Table 58.3). (4) Supplemental laboratory criteria include albumin <3 g/dL, anemia for age, elevation of alanine aminotransferase, platelets after 7 days >450,000/mm3, white blood cell count ≥15,000/mm3, and urine ≥10 white blood cells/HPF. (5) These patients can be treated before performing echocardiography. (6) Echocardiography is considered positive for purposes of this algorithm if any of three conditions are met: z-score of LAD or RCA ≥2.5, coronary arteries meet Japanese Ministry of Health criteria for aneurysms, or more than three other suggestive features exist including perivascular brightness, lack of tapering, decreased LV function, mitral regurgitation, pericardial effusion, or z-scores in LAD or RCA of 2 to 2.5. (7) If the echocardiogram is positive, treatment should be given to children within 10 days of fever onset and those beyond day 10 with clinical and laboratory signs (CRP, ESR) of ongoing inflammation. (8) Typical peeling begins under nail bed of fingers and then toes. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; f/u, follow-up. (From Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–2771, with permission.) |
Clinical Features: Cardiovascular
During the acute phase, patients may manifest signs of myocarditis, such as sinus tachycardia out of proportion to the fever, gallop rhythm, and sometimes muffled heart tones. These findings are generally self-limited and improve with IVIG treatment, although overt heart failure may occur occasionally. Levels of pro-B-type natriuretic peptide (proBNP) and troponin I (TnI) can be elevated in the acute phase (71,72), although the added value of these biomarkers in clinical care is not yet established (73,74,75). A pericardial effusion by echocardiography is not uncommon, but the effusions generally measure <1 mm (72); pericardial tamponade is very rare (76). Systolic murmurs are often heard owing to increased cardiac output and anemia, and approximately one-quarter of patients have mitral insufficiency (77). Children occasionally present with low cardiac output shock; little is known about the risk factors for this presentation although one study suggests an association with gastrointestinal symptoms (78).
Coronary artery dilation and enhanced perivascular brightness are noted in 30% to 50% of patients during the acute phase of KD. Without IVIG treatment, these lesions may become aneurysmal 1 to 3 weeks from onset of illness (average 10 days) (Fig. 58.1). The reported rates of aneurysm vary based on the definition of an aneurysm used. The Japanese National Kawasaki Disease surveillance data estimate the incidence of coronary artery dilation at 7.26%, aneurysms at 1.04%, and giant aneurysms at 0.24% (5). Analysis of the Pediatric Health Information System, an administrative database of freestanding children’s hospitals, estimates the rates of aneurysms to be between 1.8% and 5.9% (79). Coronary artery aneurysms tend to develop most frequently in the proximal segments of the left anterior descending and the RCA, and less commonly in the left main coronary artery. The left circumflex branch is least often involved. An aneurysm in the distal arterial segment is usually but not always accompanied by an aneurysm in the proximal segment of the same artery. Aneurysms with internal diameters >8 mm or a z-score of ≥10 (the so-called giant aneurysms) present disproportionately higher risks of myocardial infarction as compared with aneurysms of smaller dimensions (80,81). Several risk scores have been formulated to predict the development of coronary artery aneurysms based on clinical and laboratory data at presentation (82,83,84,85,86). Independent predictors include protracted fever, presumably reflecting worse vasculitis, anemia, elevated white blood count, low albumin, elevated CRP, male gender, age younger than 1 year or older than 9 years, and the largest baseline coronary artery dimension z-score (87).
Rarely, KD patients experience myocardial infarctions. Myocardial infarctions present differently in childhood compared to adults, with the most common symptoms being shock, chest pain, vomiting, inconsolable crying, and abdominal pain; the authors are aware of one case of referred left otalgia as a presentation of myocardial ischemia in a toddler with KD. Chest pain is reported much less frequently in children younger than 4 years of age. Approximately one-third of the patients are asymptomatic at the time of infarction, which often occurs at rest or during sleep, and infrequently during exertion (88). Fatality associated with the first episode of myocardial infarction has been reported to be 22%, with progressively worsening mortality rates with subsequent attacks. Giant aneurysms are associated with higher rates of mortality. Rarely, aneurysms may rupture and cause sudden death. In general, late consequences of KD are isolated to cardiovascular disease and occur only in patients who had coronary disease at presentation. Fortunately, overall survival rates at 10, 20, and 30 years after the onset of KD are good, reported at 95%, 88%, and 88%, respectively (89).
Electrocardiographic Features
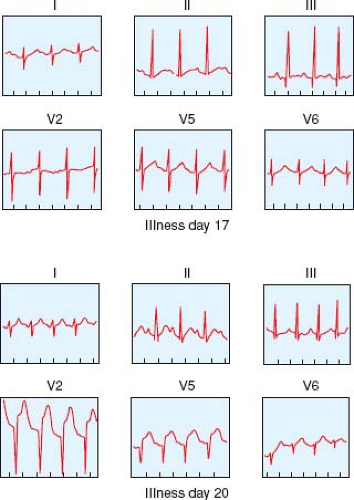
During the acute phase of KD, the electrocardiogram (ECG) may show sinus tachycardia, prolongation of PR and corrected QT intervals, decreased QRS voltage, and T-wave flattening (90). The presence of large aneurysms may lead to acute myocardial infarction manifested as ST-segment elevation and inversion of T waves (Fig. 58.1). Thrombosis of an RCA aneurysm may produce a silent posterior wall infarction manifested as abnormally deep Q waves in leads II, III, and aVF.
Radiologic Features
The chest radiograph is usually unremarkable, although transient cardiomegaly is seen in 20% of cases during the acute phase. Rarely, the chest x-ray may show localized pulmonary infiltration or pleural effusion. Patients whose coronary aneurysms persist ≥1 year after the onset of the disease may show a thin, eggshell-like calcification outlining the aneurysms.
Echocardiographic Features
Echocardiography is essential in the evaluation and management of KD. It is invaluable for detecting coronary artery aneurysms during the acute stage and should be performed at diagnosis to establish a baseline and in some cases to aid in diagnosis (Fig. 58.3), although treatment should not be delayed in patients with a clear KD diagnosis while echo imaging is obtained. Findings of perivascular brightness, mild coronary artery ectasia, and lack of tapering of the coronary arteries are relatively common in the acute stage of KD (91). Decreased left ventricular contractility (20% of acute KD), diastolic dysfunction, mild valvular regurgitation (most commonly, mitral regurgitation in 25% of acute KD), and minor pericardial effusion may also be seen on echocardiography in acute KD (77,92). Myocardial dysfunction is associated with a greater risk of coronary artery dilation (77). Aortic regurgitation occurs rarely during the chronic phase (93). Echocardiography is usually repeated at 2 and 6 weeks after the onset of illness to see the extent of coronary involvement and to guide therapy, although new abnormalities are unlikely to be detected at 6 weeks in uncomplicated patients with normal coronary arteries at baseline and at 2 weeks (94). Echocardiograms may be done more frequently in patients who have a more complicated clinical course to help guide treatment. For patients with giant aneurysms, we perform echocardiograms twice weekly early in the illness, then weekly through the first 45 days of illness, monthly until the 3rd month, and then every 3 months for the 1st year to assess for thrombosis. For long-term cardiac follow-up, echocardiography is useful for evaluating global left ventricular function, regional wall motion characteristics, and competency of mitral and aortic valves. Proximal segments of the right and left coronary arteries may be visualized in nearly all patients. Visualization of distal coronary artery segments may be technically demanding, necessitating patient sedation, use of special views (95), and careful optimization of machine settings.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree