INDICATIONS
 The most common indication for an Ivor Lewis esophagectomy is middle-third esophageal squamous or adenocarcinoma
The most common indication for an Ivor Lewis esophagectomy is middle-third esophageal squamous or adenocarcinoma
 Esophageal disorders requiring removal of most of the esophagus
Esophageal disorders requiring removal of most of the esophagus
 Distal esophageal tumors with proximal extension above 35 cm
Distal esophageal tumors with proximal extension above 35 cm
 High-grade dysplasia in Barrett’s esophagus with proximal extension above 35 cm
High-grade dysplasia in Barrett’s esophagus with proximal extension above 35 cm
 Failed myotomy for achalasia with sigmoid esophagus requiring near-total esophagectomy
Failed myotomy for achalasia with sigmoid esophagus requiring near-total esophagectomy
 CONTRAINDICATIONS
CONTRAINDICATIONS
Contraindications to an Ivor Lewis esophagectomy are relative and in some cases may include prior thoracotomy, especially for inflammatory disease on the right side. Factors to be considered include the nature of esophageal disease (benign vs. malignant), the need for adequate longitudinal and radial margins, surgeon preference, patient factors, and neoadjuvant treatment.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
Careful evaluation of each patient is essential. Patients must be in reasonable medical condition and must have adequate pulmonary function. Patients with forced expiratory volume in 1 second of less than 1 L are probably not suitable candidates for this approach. Smoking should be stopped, and aggressive measures to treat underlying obstructive lung disease should be instituted. Careful evaluation for underlying cardiac disease should be done in elderly and high-risk patients.
Radiologic Evaluation
Standard preoperative evaluation includes the following.
 Barium esophagography
Barium esophagography
 Computed tomography (CT) of the chest and upper abdomen
Computed tomography (CT) of the chest and upper abdomen
 For malignant esophageal tumors, esophagoscopy with endoscopic ultrasound to assess depth of invasion and presence of enlarged lymph nodes30
For malignant esophageal tumors, esophagoscopy with endoscopic ultrasound to assess depth of invasion and presence of enlarged lymph nodes30
Endoscopic Evaluation
Histologic diagnosis and determination of the true proximal and distal extent of the tumor is best achieved through endoscopic evaluation. Direct visualization also allows for checking of the presence of Barrett’s mucosa proximal to adenocarcinomas. The proximal extent of the tumor and abnormal mucosa is critical in determining surgical approach.
Endoscopy should be performed by the surgeon in all cases. It is particularly helpful in determining proximal extent of tumor. A 5-cm surgical margin is desirable for carcinoma. Distal tumors with proximal involvement above 35 cm may be technically difficult to resect from the left side. Tumor involvement of the esophagus between 30 and 35 cm can be approached from either the left side, utilizing a supra-aortic anastomosis, or the more traditional Ivor Lewis approach.
In patients with adenocarcinoma arising in Barrett’s mucosa, it is important to resect the tumor, with a 5-cm proximal margin and all of the Barrett’s mucosa. It is helpful to identify the proximal extent of Barrett’s mucosa by endoscopically placing the nasogastric tube just above the squamocolumnar junction or by performing endoscopy intraoperatively to identify the location. Tumors extending above 30 cm may involve either the left main stem bronchus or the trachea and should be evaluated with bronchoscopy by the surgeon.
A combined thoracoscopy–laparoscopy, where peritoneal or pleural seeding, lymph node involvement, and local extent of tumor can all be assessed offers the potential for minimally invasive surgical staging of esophageal cancer and is an adjunct similar to that associated with mediastinoscopy for lung cancer.14,20 Pretreatment staging is necessary to better evaluate response to neoadjuvant therapy and should include esophageal ultrasound, CT scan, PET scan, and brain MRI.
Open Ivor Lewis Esophagectomy
 SURGERY
SURGERY
Abdominal Phase
Positioning
With the patient in the supine position, an upper midline abdominal incision is made (Fig. 21.1). The abdomen is explored. If liver metastases or unresectable retroperitoneal nodes are found, resection should be abandoned, and palliation of dysphagia should be achieved by other means. Endoscopically placed covered esophageal stents or irradiation have successfully been used.8,32
Technique
 If the tumor is resectable, the left triangular ligament of the liver is divided.
If the tumor is resectable, the left triangular ligament of the liver is divided.
 The lesser sac is entered through the greater omentum, preserving the gastroepiploic artery.
The lesser sac is entered through the greater omentum, preserving the gastroepiploic artery.
 The omentum is separated from the transverse colon, and care taken to preserve the gastroepiploic artery. If possible, omentum along the greater curvature is saved for later use to cover the anastomosis. Excess bulk of omentum can be removed to facilitate transport into the chest. By elevating the greater curvature of the stomach, while preserving the gastroepiploic artery, the surgeon can easily divide the short gastric vessels (Fig. 21.2).
The omentum is separated from the transverse colon, and care taken to preserve the gastroepiploic artery. If possible, omentum along the greater curvature is saved for later use to cover the anastomosis. Excess bulk of omentum can be removed to facilitate transport into the chest. By elevating the greater curvature of the stomach, while preserving the gastroepiploic artery, the surgeon can easily divide the short gastric vessels (Fig. 21.2).
 The gastrohepatic ligament is divided taking care to preserve the right gastric artery.
The gastrohepatic ligament is divided taking care to preserve the right gastric artery.
 The left gastric artery and vein are isolated and doubly suture-ligated at their origin with lymph nodes taken from this area with the specimen.
The left gastric artery and vein are isolated and doubly suture-ligated at their origin with lymph nodes taken from this area with the specimen.
 The hiatus and distal esophagus are dissected. Enlarging the hiatus from the right chest is difficult and more easily achieved through the laparotomy incision.
The hiatus and distal esophagus are dissected. Enlarging the hiatus from the right chest is difficult and more easily achieved through the laparotomy incision.
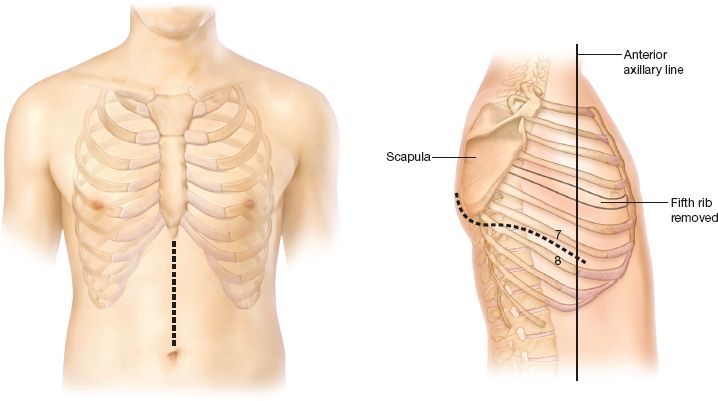
Figure 21.1 Standard incisions for an Ivor Lewis esophagectomy. An upper midline abdominal incision is used to mobilize the stomach. A right thoracotomy is used to resect the esophagus and do the esophagogastric anastomosis.

Figure 21.2 The omentum is separated from the transverse colon and care taken to preserve the gastroepiploic artery. If possible, omentum along the greater curvature is saved for later use to cover the anastomosis. Excess bulk of omentum can be removed to facilitate transport into the chest.
 Maximal mobility of the stomach can be achieved with a Kocher maneuver.
Maximal mobility of the stomach can be achieved with a Kocher maneuver.
 A pyloroplasty or pyloromyotomy is often done at the surgeon’s discretion.
A pyloroplasty or pyloromyotomy is often done at the surgeon’s discretion.
 It is helpful to identify the point of transection of the stomach and clear the greater and lesser curvature in the abdomen. At this point, the final diameter of the new conduit must be determined; there are arguments in favor of a wider conduit and in favor of a narrow conduit. No objective data exist in terms of the ideal diameter of the new conduit. One argument in favor of a narrow conduit (3 to 4 cm in diameter) is that it may lead to less acid reflux.
It is helpful to identify the point of transection of the stomach and clear the greater and lesser curvature in the abdomen. At this point, the final diameter of the new conduit must be determined; there are arguments in favor of a wider conduit and in favor of a narrow conduit. No objective data exist in terms of the ideal diameter of the new conduit. One argument in favor of a narrow conduit (3 to 4 cm in diameter) is that it may lead to less acid reflux.
 A feeding jejunostomy is inserted in most patients, especially high-risk or nutritionally depleted patients.
A feeding jejunostomy is inserted in most patients, especially high-risk or nutritionally depleted patients.
It is helpful to accomplish as much dissection of the lower esophagus as possible from the abdomen. This dissection facilitates the intrathoracic dissection of the lower esophagus, which can be difficult through a high right thoracotomy. An attempt should be made to advance the stomach and omentum into the posterior omentum before closing the abdomen to facilitate retrieval of the conduit from the chest.
Thoracic Phase
Positioning
The patient is then placed in the left lateral decubitus position. A double-lumen endotracheal tube is used allowing the lung to collapse and exposing the esophagus for dissection and anastomosis.
Technique
A standard posterolateral right thoracotomy is performed. The serratus muscle is spared, and the chest is entered through the fourth or the fifth interspace. After the lung is examined for abnormalities, it is deflated and retracted anteriorly.
 The azygos vein is divided.
The azygos vein is divided.
 The esophagus is dissected from the vertebral body to the pericardium. All paraesophageal nodes, including subcarinal nodes, are included in the specimen. Dissection is continued to the apex of the chest.
The esophagus is dissected from the vertebral body to the pericardium. All paraesophageal nodes, including subcarinal nodes, are included in the specimen. Dissection is continued to the apex of the chest.
 The stomach is then pulled into the chest and divided. It is done only after all of the intrathoracic dissection has been completed to avoid any confusion about orientation of the stomach.
The stomach is then pulled into the chest and divided. It is done only after all of the intrathoracic dissection has been completed to avoid any confusion about orientation of the stomach.
 Care must be taken to avoid pulling too much of the stomach into the chest. Redundant gastric conduit above the diaphragm will fall into the costophrenic gutter, creating an S-loop and cause delayed emptying of the stomach.
Care must be taken to avoid pulling too much of the stomach into the chest. Redundant gastric conduit above the diaphragm will fall into the costophrenic gutter, creating an S-loop and cause delayed emptying of the stomach.
 Pulling the stomach too tightly into the chest may also lead to impingement of the stomach at the level of the hiatus and cause delayed emptying as well as compression of the gastroepiploic vessels.
Pulling the stomach too tightly into the chest may also lead to impingement of the stomach at the level of the hiatus and cause delayed emptying as well as compression of the gastroepiploic vessels.
 It is best to grasp the omentum near the stomach to avoid tearing the gastroepiploic vessels.
It is best to grasp the omentum near the stomach to avoid tearing the gastroepiploic vessels.
Anastomotic Technique
Sweet35 published his initial experience with surgical management of carcinoma of the esophagus in 141 patients in 1947. Operating in an era without sophisticated postoperative monitoring devices, mechanical ventilation, or broad-spectrum antibiotics, his results were remarkable: An operative mortality of 15%, anastomotic leaks in 1.4% of patients, and overall 5-year survival of 11%. These results served as a standard for many years. The low incidence of leaks and operative mortality was related to the attention to detail and the reliability of the anastomotic technique.
 Churchill and Sweet6 warned of factors predisposing to anastomotic leak-namely the lack of an esophageal serosal layer and the segmental blood supply of the esophagus.
Churchill and Sweet6 warned of factors predisposing to anastomotic leak-namely the lack of an esophageal serosal layer and the segmental blood supply of the esophagus.
 Churchill and Sweet emphasized the importance of preserving the esophageal and gastric blood supply; gentle handling of the tissues, interrupted sutures, avoiding crushing clamps, cutting with a knife or other sharp instrument, and firm but gentle tying of sutures to avoid cutting tissues.
Churchill and Sweet emphasized the importance of preserving the esophageal and gastric blood supply; gentle handling of the tissues, interrupted sutures, avoiding crushing clamps, cutting with a knife or other sharp instrument, and firm but gentle tying of sutures to avoid cutting tissues.
The traditional anastomotic technique is as follows.
 A 2-cm diameter circle is scored on the surface of the stomach (Fig. 21.3A). The defect created should be 2 cm away from the stapled edge of the stomach to avoid a compromised blood supply.
A 2-cm diameter circle is scored on the surface of the stomach (Fig. 21.3A). The defect created should be 2 cm away from the stapled edge of the stomach to avoid a compromised blood supply.
 Submucosal vessels are identified and individually ligated with fine silk sutures.
Submucosal vessels are identified and individually ligated with fine silk sutures.
 Interrupted mattress sutures of fine suture (4-0 silk) are used to construct the back row of the anastomosis (Fig. 21.3B). Corner stitches are placed first, with the remaining sutures placed evenly between them. The sutures on the stomach include the seromuscular layers. The esophageal sutures should be deep enough to include both the longitudinal and circular muscular layers of the esophagus.
Interrupted mattress sutures of fine suture (4-0 silk) are used to construct the back row of the anastomosis (Fig. 21.3B). Corner stitches are placed first, with the remaining sutures placed evenly between them. The sutures on the stomach include the seromuscular layers. The esophageal sutures should be deep enough to include both the longitudinal and circular muscular layers of the esophagus.
 The esophagus is opened sharply from one corner stitch to the other. The circular button of the stomach is removed (Fig. 21.3B).
The esophagus is opened sharply from one corner stitch to the other. The circular button of the stomach is removed (Fig. 21.3B).
 The inner layer is completed with simple sutures, including just the mucosa of the esophagus and all layers of the stomach (Fig. 21.3C).
The inner layer is completed with simple sutures, including just the mucosa of the esophagus and all layers of the stomach (Fig. 21.3C).
 The knots are on the inside, allowing inversion or turning in of mucosa of both the stomach and the esophagus. This step is accomplished for the entire circumference of the anastomosis (Fig. 21.3D).
The knots are on the inside, allowing inversion or turning in of mucosa of both the stomach and the esophagus. This step is accomplished for the entire circumference of the anastomosis (Fig. 21.3D).
 A nasogastric tube is passed into the stomach under direct vision before a single Connell stitch is placed for closure of the final opening. Healing of the inverted mucosa is important in preventing leakage, and the location of the knots on the luminal side minimizes foreign-body reaction with the actual tissues of the anastomosis.
A nasogastric tube is passed into the stomach under direct vision before a single Connell stitch is placed for closure of the final opening. Healing of the inverted mucosa is important in preventing leakage, and the location of the knots on the luminal side minimizes foreign-body reaction with the actual tissues of the anastomosis.
 The outer row is completed using horizontal mattress sutures as described for the back row of the outer layer (Fig. 21.3E).
The outer row is completed using horizontal mattress sutures as described for the back row of the outer layer (Fig. 21.3E).
 The omentum, mobilized with the stomach, is placed over the anastomosis anteriorly to provide an additional layer of coverage. In order to avoid tension on the anastomosis when the patient is upright, several sutures are placed between the stomach and the mediastinal pleura.
The omentum, mobilized with the stomach, is placed over the anastomosis anteriorly to provide an additional layer of coverage. In order to avoid tension on the anastomosis when the patient is upright, several sutures are placed between the stomach and the mediastinal pleura.
 Sutures are also placed between the stomach and the diaphragmatic hiatus to prevent herniation of abdominal contents.
Sutures are also placed between the stomach and the diaphragmatic hiatus to prevent herniation of abdominal contents.
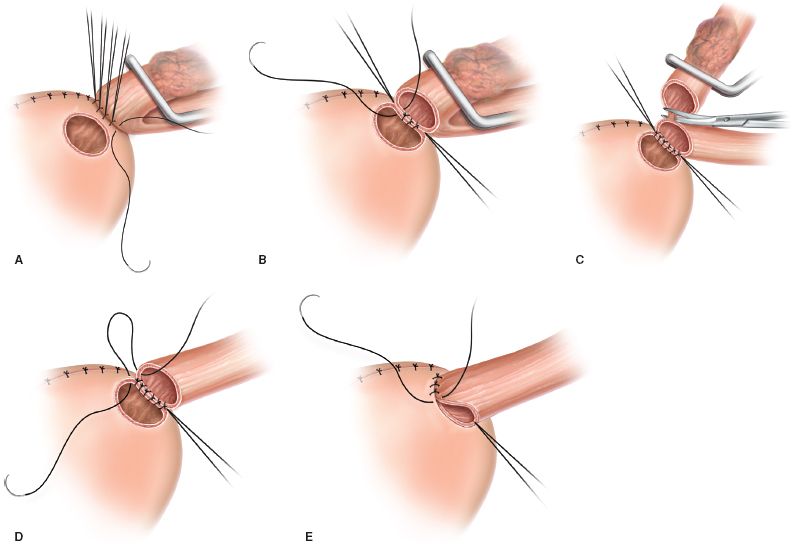
Figure 21.3 Surgical technique. A: The serosa of the stomach has been scored, and the vessels have been ligated. The back row of sutures has been completed. B: The button of stomach has been removed, and the anterior wall of the esophagus has been opened. C: The back row of the inner layer is completed, and the esophagus is transected. D: Knots on the inside allow inversion of the mucosa. E: The outer row is completed using horizontal mattress sutures.
In accordance with Dr. Churchill and Sweet’s teachings, trauma to the tissues is avoided as much as possible. Once the first stitch is placed and tied, traction on it permits placement of the next without the need of instrumental grasping of the mucosa. The surgeon ties the sutures by positioning the index finger cephalad to the anastomosis and lifting the stomach to the esophagus. This avoids pulling down on the fixed and more fragile esophagus.
A nasogastric tube passed through the anastomosis for a short time decompresses the stomach and avoids distention of the suture line. Gentle, periodic irrigation of the tube ensures its patency. Temporary gastric decompression more than compensates for any potentially deleterious effect of the intraluminal foreign body lying against the suture line for a short period.
 COMPLICATIONS
COMPLICATIONS
Experience with this technique from the Massachusetts General Hospital in a consecutive series of 104 patients was reported.22 There were three postoperative deaths (2.9%), all attributable to pneumonia and respiratory failure. Five patients developed anastomotic stricture requiring dilation 3 to 6 weeks postoperatively. Dysphagia resolved after one to three dilations. Delayed anastomotic stricture was not present in this group of patients. All patients underwent a postoperative barium swallow. There were no anastomotic leaks, even of the localized type. The reliability of this precise, two-layer anastomotic technique has been reported by others as well.7
Anastomotic Leaks
Aggressive management of anastomotic leaks is required if fatalities are to be avoided.
 If the leak is small and contained or well drained by the chest tube, the patient should take nothing by mouth; antibiotic therapy and nutritional support should be continued, and the contrast study should be repeated 1 week later.
If the leak is small and contained or well drained by the chest tube, the patient should take nothing by mouth; antibiotic therapy and nutritional support should be continued, and the contrast study should be repeated 1 week later.
 CT is performed to identify any undrained collections.
CT is performed to identify any undrained collections.
 Small, undrained collections can be drained by percutaneous ultrasound-guided catheters.
Small, undrained collections can be drained by percutaneous ultrasound-guided catheters.
The presence of a massive leak warrants urgent intervention. If the leak is related to necrosis of the stomach, the stomach should be resected to viable tissue, returned to the abdomen, and a cervical esophagostomy should be done. Reconstruction of the gastrointestinal tract can be performed at a later date. Local repair in the presence of gross contamination of the pleural cavity can be expected to fail in most cases.
 If local repair is attempted, devitalized tissue should be debrided and the repair should be buttressed with healthy tissue. Omentum, chest wall muscles (serratus, pectoralis), or pedicled intercostal muscle can all be used.
If local repair is attempted, devitalized tissue should be debrided and the repair should be buttressed with healthy tissue. Omentum, chest wall muscles (serratus, pectoralis), or pedicled intercostal muscle can all be used.
 The lung should be decorticated, and wide drainage of the pleural cavity should be provided.
The lung should be decorticated, and wide drainage of the pleural cavity should be provided.
 Cervical esophagostomy may be appropriate if concern exists about the repair. The esophagostomy can be constructed in such a way that reanastomosis can be done between the divided ends of the esophagus in the neck, avoiding more complicated reconstructive methods.10
Cervical esophagostomy may be appropriate if concern exists about the repair. The esophagostomy can be constructed in such a way that reanastomosis can be done between the divided ends of the esophagus in the neck, avoiding more complicated reconstructive methods.10
 Alternatively if preservation of the conduit is desired and the anastomosis is otherwise intact, a long T-tube can be placed through the defect, brought out through the chest wall with pedicled muscle wrapped around the T-tube fistula. Drains should be placed in close proximity to T-tube.
Alternatively if preservation of the conduit is desired and the anastomosis is otherwise intact, a long T-tube can be placed through the defect, brought out through the chest wall with pedicled muscle wrapped around the T-tube fistula. Drains should be placed in close proximity to T-tube.
Delayed Gastric Emptying
The two main reasons for delayed gastric emptying after an Ivor Lewis esophagectomy are the following.
 Obstruction at the hiatus
Obstruction at the hiatus
 Redundant intrathoracic stomach lying in the posterior costophrenic gutter
Redundant intrathoracic stomach lying in the posterior costophrenic gutter
These problems are best avoided by an adequate drainage procedure at the time of operation, with the surgeon enlarging the hiatus, not pulling the stomach too tightly into the chest, and avoiding excess stomach in the chest.
Pyloric obstruction may also cause delayed gastric emptying. When a drainage procedure has been done, pyloric obstruction usually resolves with time. Metoclopramide may be useful. Endoscopy and cautious balloon dilation of the pylorus can be tried. Failure of conservative management requires reoperation and an adequate drainage procedure. Obstruction at the level of the hiatus usually demands reexploration and enlargement of the hiatus. This procedure is often difficult, and great care must be taken to avoid injury to the blood supply of the stomach.
Mortality
Advances in anesthesia, perioperative care, monitoring, and enteral and parenteral nutrition have resulted in a dramatic decrease of 50% over 10 years in overall postoperative mortality. In addition, there was a significantly lower hospital mortality rate for resections performed with curative intent versus those with palliative intent (mean, 11% vs. 19%, respectively).24 The hospital mortality rate was nearly identical whether or not the anastomosis was located in the neck if the procedure involved a thoracotomy of any type. Many institutions have reported operative mortality rates near or below 5% for either transthoracic or transhiatal resection.3,29,37,38
Survival
Despite the theoretical advantages of an en bloc dissection and full lymphadenectomy, studies comparing these procedures with transhiatal esophagectomy show no difference in overall survival between these two approaches for esophageal carcinoma. Shahian et al.31 demonstrated no statistically significant difference in survival for all patients who underwent transthoracic versus extrathoracic esophagectomy for carcinoma (median, 14.1 vs. 12.6 months; p = 0.48), regardless of whether patients had stage I or stage III disease. In the review by Müller, only tumor stage at time of operation was a significant determinant of long-term survival. There was no significant difference in survival according to extent of surgery or type of resection.
 RESULTS
RESULTS
The greatest immediate concern is the fate of the intrathoracic anastomosis. It is undoubtedly this concern that has led to the popularity of the transhiatal esophagectomy, which places the anastomosis in the cervical area.28 We and others have shown that attention to technical details of the anastomosis leads to a very low incidence of leaks and subsequent mortality, stressing the importance of how the anastomosis is done rather than where it is done.
Transhiatal esophagectomy has become the popular alternative to Ivor Lewis esophagectomy. No direct randomized series have compared the two procedures. Müller et al.24 reviewed all published reports of surgical therapy of esophageal carcinoma from 1980 to 1988 (a summary of 59 published reports) and drew some conclusions about the two procedures. Others have compared the two procedures in a retrospective fashion within a single institution and provide some important insights into the relative merits of the two procedures.3,17
In most reports, the risk of anastomotic leak from a cervical anastomosis is higher than that reported for an intrathoracic anastomosis.24 In patients experiencing leaks, however, morbidity and mortality related to the leak itself are lower if the anastomosis is in the neck rather than in the chest. This finding is borne out in the Müller review. The anastomotic leak rate was 11% for intrathoracic anastomosis compared with 19% for cervical anastomosis. However, the mortality rate for an intrathoracic anastomotic leak was three times higher than that for a cervical anastomotic leak (69% vs. 29%, respectively).
Cervical anastomotic leak rates for transhiatal esophagectomy without thoracotomy have been reported to be as low as 6% to 8% for operations performed for carcinoma of the intrathoracic esophagus,28 and leak rates from 0% to 2% have been reported for intrathoracic anastomosis.9,22,38 Even within the same institution and with the same surgeons, cervical anastomosis is associated with a higher incidence of leaks. This finding is confirmed by a report from the Lahey Clinic, with 15.4% leak for cervical anastomosis but 1.8% for intrathoracic anastomosis.31
Respiratory insufficiency and atelectasis occur more commonly after transthoracic esophagectomy, but the incidence of pneumonia is similar. Transhiatal esophagectomy is associated with a high incidence of recurrent nerve paresis or palsy (6% to 24%), but this is very uncommon after transthoracic esophagectomy. Chylothorax, posterior membranous tracheal tears, and increased blood loss are all more frequent after transhiatal esophagectomy. Most reports have not demonstrated the superiority of one surgery over another and view the procedures as alternative choices.17,31
In a recent presentation at the American Surgical Association annual meeting, a national database of over 15,000 esophagectomies was evaluated. The primary comparison was between patients having an intrathoracic anastomosis versus a cervical anastomosis. The interesting findings of this study were that the patients with the intrathoracic anastomosis fared markedly better in terms of length of stay, mortality, and hospital charges. Another interesting finding was that the leak rate was about 10% in each group, but overall morbidity and mortality still favored the intrathoracic anastomosis group.11 This data compare favorably with the recent series by Luketich et al.18 where they compared ~500 consecutive neck anastomosis to ~500 intrathoracic anastomosis and found better outcomes with the intrathoracic anastomosis as well So, it does appear that over the last decade or so, we are seeing an increasing number of surgeons choose the Ivor Lewis approach with morbidity that is quite favorable compared with cervical anastomosis reports.
Late Functional Results
Although little information has been reported about late functional results after an Ivor Lewis esophagectomy, the Mayo clinic did report early and late functional results in 100 patients.12 A pyloromyotomy (39 patients) or pyloroplasty (56 patients) was done in 95 patients. Early functional results were excellent, with development of dysphagia and gastroesophageal reflux in only 3% and 1% of patients, respectively. The mean follow-up of patients was 2.3 years. Late dysphagia occurred in 40 patients; in 5 patients it was related to anastomotic recurrence and in 35 to benign anastomotic narrowing, requiring dilation. Dilation (range, 1 to 22 dilations; mean, 3.4 dilations) relieved symptoms in all 35 patients with benign stenosis. Delayed presentation of reflux occurred in 14% of patients and dumping in 5%. All patients with reflux or dumping were treated successfully by standard medical therapy. Postoperative weight loss (median, 15.7 kg) occurred in 62% of patients. The authors believed that weight loss was multifactorial and not necessarily related to the procedure itself.
 CONCLUSIONS
CONCLUSIONS
Ivor Lewis esophagectomy remains an excellent procedure for resection of the middle third of the esophagus with good long-term functional results. Proper patient selection, adequate preoperative preparation of the patient, attention to technical details of the operation, and diligent postoperative care allow this procedure to be performed safely with acceptable morbidity and mortality rates. A reliable anastomotic technique should be implemented to avoid intrathoracic anastomotic leaks, the source of greatest morbidity and mortality. Ivor Lewis esophagectomy offers potential advantages of wider, more complete resection for better staging information, lower local recurrence rates, and possibly improved survival as compared with transhiatal esophagectomy.
Minimally Invasive Esophageal Resection
More recently, in an effort to limit the physiologic stress and possibly reduce the morbidity associated with open esophagectomy, minimally invasive surgical approaches to esophagectomy have been developed.21 The dramatic improvement in laparoscopic technology since its introduction in 1991 has witnessed an associated evolution in the complexity of laparoscopic technology. Complex esophageal disorders, including achalasia and giant paraesophageal hernia, are being successfully treated laparoscopically. Watson et al.40 reported a minimally invasive Ivor Lewis technique in 1999 with their description of a laparoscopically constructed gastric conduit followed by thoracoscopic esophagectomy with construction of an intrathoracic esophagogastric anastomosis.
Concerns in minimally invasive esophagectomy include the amount of nodal clearance achieved, the complexity of the procedure, and the ability to achieve a measurable impact on mortality. Early data reporting the outcomes of minimally invasive esophagectomy challenge these concerns. Recent studies have shown that minimally invasive esophagectomy is associated with outcomes comparable with open surgery.4,15,19,27,33 The minimally invasive Ivor Lewis esophagectomy is also described in detail in chapter 24.
Minimally Invasive Ivor Lewis Esophagectomy
 SURGERY
SURGERY
Abdominal Phase
Positioning
The patient is positioned supine and a double-lumen tube is placed for eventual lung isolation. As with the open technique, on table esophagoscopy is performed to determine the location and extent of tumor involvement as well as to assess the stomach for suitability as a gastric conduit.
 Five ports are placed in the abdomen for gastric mobilization (Fig. 21.4).
Five ports are placed in the abdomen for gastric mobilization (Fig. 21.4).
 The liver and peritoneal surfaces are inspected to rule out metastatic disease.
The liver and peritoneal surfaces are inspected to rule out metastatic disease.
 The celiac and left gastric artery and vein lymph nodes are identified. Any suspicious nodes are removed and sent for frozen section analysis.
The celiac and left gastric artery and vein lymph nodes are identified. Any suspicious nodes are removed and sent for frozen section analysis.
Technique
Dissection begins with mobilization of the right crus and the lateral aspect of the esophagus. The dissection is carried anteriorly and superiorly over the esophagus toward the left crus. Division of the phrenoesophageal ligament is spared until the end of the case in order to maintain pneumoperitoneum. A retroesophageal window is created through dissection of the inferior aspect of the right crus.
 The lesser sac is then entered through cephalad retraction of the antrum of the stomach taking care to preserve the right gastroepiploic artery.
The lesser sac is then entered through cephalad retraction of the antrum of the stomach taking care to preserve the right gastroepiploic artery.
Using ultrasonic shears (Autosonix, Covidien, Mansfield, MA; Harmonic Scalpel, Johnson and Johnson, Piscataway, NJ) and the Ligasure device (Ligasure, Valleylab, Boulder, CO), dissection is carried along the greater curvature of the stomach to the end of the gastroepiploic arcade with division of the short gastric vessels. Once mobilization of the greater curvature of the stomach is achieved, the retrogastric attachments are exposed by lifting the fundus of the stomach toward the patient’s right shoulder.
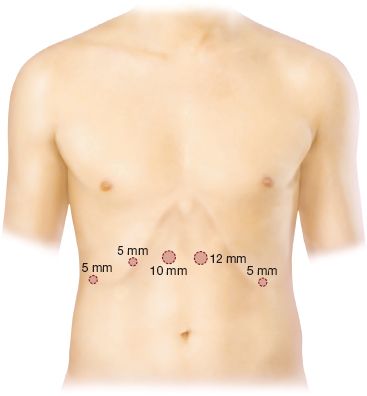
Figure 21.4 Five ports are placed in the abdomen for gastric mobilization.



