Isolated Thoracic Aortic Aneurysm: TEVAR
JULIA SARAIDARIDIS and MARK CONRAD
Presentation
A 70-year-old male with a past medical history of hypertension and smoking is referred to your office for discussion of a descending thoracic aortic aneurysm (DTA) that was identified as an incidental finding on a chest computed tomography (CT) scan obtained during an emergency department workup of a minor motor vehicle accident. He denies any history of chest or back pain. His past medical and surgical history is remarkable for mild COPD, a left hip replacement, hyperlipidemia (on statin therapy), and hypertension that is well controlled on two agents. He is a former smoker with a 40 pack-year history. On physical examination, he has normal vital signs, clear lungs, a soft abdomen, and a normal pulse exam. His labs show no significant findings, and the CT scan he brings to clinic shows an aneurysmal dilation of the descending thoracic aorta with greatest diameter of 7.0 cm by center-line measurement.
Differential Diagnosis
The majority of DTAs are asymptomatic and are often identified as incidental findings on imaging studies obtained for other indications. On the other hand, a ruptured, expanding, thoracic aortic aneurysm will often present as an acute aortic syndrome whose symptoms include sudden, sharp, tearing chest or back pain. The experience of these symptoms usually prompts the patient to seek emergent medical attention. The differential diagnosis of acute aortic syndrome is a ruptured/expanding aortic aneurysm, acute aortic dissection, aortic intramural hematoma, penetrating aortic ulcer, myocardial infarction, pulmonary embolism, or pneumothorax. For a patient in whom a DTA is discovered incidentally on imaging, the differential diagnosis includes a variety of aortic pathologies including aortic dissection, ascending aortic aneurysm, or thoracoabdominal aortic aneurysm.
Workup
All patients in whom endovascular repair of a thoracic aortic aneurysm (TEVAR) is considered should undergo a CT angiography (CTA) of the chest, abdomen, and pelvis with fine (≤5 mm) cuts. From this scan, a three-dimensional model can be constructed to aid in preprocedural planning (Fig. 1). The chest portion of this study provides information regarding the aortic arch anatomy, the diameter and length of potential seal zones, the relationship of the aneurysm to the left subclavian and celiac arteries, and the presence of intraluminal thrombus. The abdominal and pelvic portions of the CTA are essential for evaluation of calcification and tortuosity of the abdominal aorta. In addition, the scan is necessary to assess adequacy of the iliac arteries as conduit vessels through which to insert large-diameter sheaths, which are necessary to deliver an endograft into the thoracic aorta (Fig. 1).
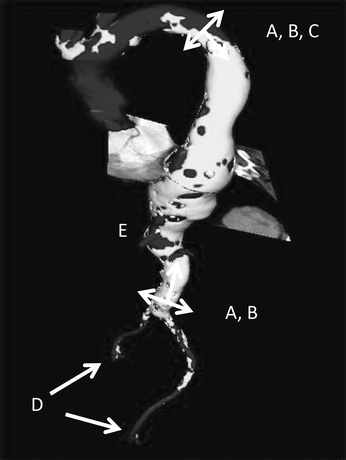
FIGURE 1 Three-dimensional reconstruction of DTA should be used for preoperative planning. Important measurements to obtain: (A)Proximal/distal seal zones length, (B)Diameter of seal zones, (C )Relationship to left subclavian/celiac artery, (D)Access vessels’ diameter/calcification, (E )Tortuosity.
Although TEVAR is much less invasive than is open repair of a thoracic aortic aneurysm, the procedure is still associated with significant morbidity and mortality. It is essential for elective patients to undergo a full preoperative assessment. Risk factors, such as coronary artery disease, renal dysfunction, and poor functional status, need to be identified prior to operation. A preoperative chest radiograph (CXR) and electrocardiogram (EKG) should be obtained in all patients. In addition, all patients should undergo a cardiac risk assessment, and, for those who are high risk, a nuclear stress test is indicated.
Diagnosis and Treatment
DTAs are rare with an incidence between 5 and 10 cases per 100,000 patient-years. They are often associated with other aortic degenerative diseases: aortitis (giant cell arteritis), genetic connective tissue disorders (Marfan’s, Ehlers-Danlos, and Loeys-Dietz syndromes), and otherwise unidentified familial predispositions to large-vessel aneurysms. When a familial predisposition to aneurysm degeneration is identified, members of that family should be closely followed with a surveillance program aimed at identifying patients prior to the point where their aneurysm has reached accepted size thresholds for elective repair. For all patients, the decision to electively repair a DTA in the hopes of preventing future rupture is dependent upon the location, size, and shape of the aneurysm. Overall, it is agreed that a thoracic aortic aneurysm expands slowly over time with the risk of rupture increasing significantly when the aneurysm reaches a diameter of 6.0 cm. Therefore, 6.0 cm has become the recommended size threshold for elective repair for patients who are of appropriate operative risk. However, patients with a symptomatic aneurysm or rapid growth of the aneurysm (>0.5 cm in 6 months) may be repaired at a smaller size.
Evolution of TEVAR
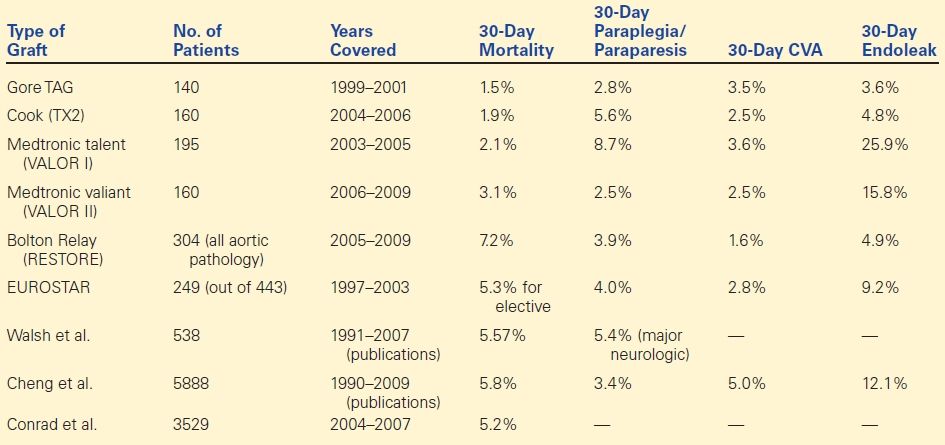
DTAs have been repaired operatively since the 1950s. In 1994, Dake et al. reported the first successful exclusion of a DTA with an endovascular device. Following this innovation, it quickly became clear that TEVAR was a viable alternative to open repair as this procedure appeared to avoid much of the significant morbidity associated with the requisite thoracotomy and aortic cross-clamping of an open repair. The Food and Drug Administration (FDA) approved the first commercially available TEVAR device in the United States in 2005, and, currently, there are five devices approved by the FDA for use in the United States for the endovascular repair of isolated DTA. Over the last 10 years, industry-sponsored device trials and large case series have provided medium- and long-term outcome data following TEVAR ( Table 1). It is clear that the perioperative mortality (3% vs. 10%) and perioperative morbidity including stroke (5% vs. 6%), paraplegia/paraparesis (5% vs. 13%), and cardiac complications (15% vs. 32%) are either equivalent or less for TEVAR than historical data for open surgical repair. As a result, for anatomically appropriate candidates, TEVAR has become the preferred method of DTA repair.
TABLE 1. List of Industry-Sponsored Trials/Sizable Case Series of TEVAR for DTA
Surgical Approach
Preoperative Planning
The key to successful exclusion of a DTA with TEVAR is in the preoperative planning. A fine-cut CTA should undergo three-dimensional modeling in order to ensure that accurate center-line measurements are obtained: several platforms exist to accomplish this task, and it is important to become facile with one of them. There are several anatomic factors that must be met in order to successfully perform TEVAR for DTA (Table 2). First, it is of utmost importance to choose appropriate proximal and distal seal zones for the graft. The aortic arch has been divided into five proximal landing zones that by virtue of their anatomic characteristics alter the ability and ease of successful deployment in that zone (Fig. 2). In general, a proximal seal in zones 0 to 2 is associated with a higher complication rate compared to zones 3 and 4. Because of the intense physiologic environment of the thoracic aorta, the length of the proximal seal is important, and although a 2-cm seal is adequate in most cases, in the face of a tortuous vessel, a longer seal zone may be necessary. Ideally, the 2-cm proximal seal zone is distal to the takeoff of the left subclavian artery (zones 3 and below). However, in 20% of patients, coverage of the left subclavian artery (zone 2) is required to obtain an adequate length of seal. In emergent situations, the left subclavian artery can be covered with minimal morbidity. However, a left carotid to subclavian bypass should be considered in patients who are at high risk of arm ischemia, spinal cord ischemia (SCI), or cerebral malperfusion (those who have undergone coronary artery bypass graft with use of the left internal mammary artery, those with a dominant left vertebral artery, those with an aberrant right subclavian artery, those with a hypoplastic right vertebral artery, those with an anomalous origin of the left vertebral artery, and those who have increased risk of paraplegia secondary to extensive coverage of the thoracic aorta). Finally, the diameter of the seal zone is currently limited to 42 mm, but grafts this size should be used with caution, as placing a graft in an aneurysmal aorta often leads to further degeneration of the aortic wall with subsequent loss of proximal seal.
TABLE 2. Anatomic Requirements for TEVAR




