Isolated Thoracic Aortic Aneurysm (Open)
GUIDO H. W. VAN BOGERIJEN and HIMANSHU J. PATEL
Presentation
A 67-year-old female with a history of tobacco use (current smoker, 50 pack-years), hypertension, depression, chronic sinusitis, history of colon cancer status post colectomy, and breast cancer with lumpectomy and radiation therapy is referred to your office by her primary care physician. The patient experienced an allergic reaction to Allegra and underwent evaluation of the chest. An x-ray demonstrated abnormal findings suggestive for a left thoracic mass. On initial questioning, the patient denies any pain or other symptoms. Her medications include loperamide, atenolol, lisinopril, aspirin, and sertraline. Physical examination reveals no abnormalities.
Differential Diagnosis
Considering patient age, history of tobacco use, and the abnormal chest x-ray, a lung mass is an important concern. To rule out a lung mass, further evaluation of the chest is indicated through CT imaging. Taking into account the abnormal chest x-ray, also a mediastinal mass should be considered. To further evaluate a possible mediastinal mass, which is most commonly of thymic or lymph node origin, further evaluation with CT imaging is warranted. An asymptomatic descending thoracic aortic aneurysm (TAA) is frequently an incidental finding detected during evaluation of the chest performed for another medical purpose. Descending thoracic aneurysms occur most commonly in the sixth and seventh decades of life, and hypertension is an important risk factor that is seen in greater than 60% of the TAA patients. To detect a TAA, total aorta CT imaging is indicated.
Workup
CT imaging should be performed to further evaluate the left thoracic mass detected on the chest x-ray. A total aorta CT scan, with thin 1-mm cuts, including the chest, abdomen, and pelvis, is performed and reveals an atherosclerotic thoracoabdominal aorta with a distal arch and descending TAA with a maximum diameter at the mid–descending aorta of 6.5 × 7.4 cm (Fig. 1). Also, the ascending aorta and abdominal aorta are dilated as demonstrated in Figure 2.
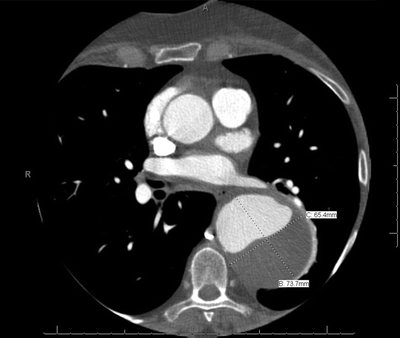
FIGURE 1 TAA with maximum diameter at the mid–descending thoracic aorta (6.5 to 7.4 cm).

FIGURE 2 3D reconstruction of the total aorta CT. TAA of the distal arch and total descending aorta is demonstrated; also dilation of the ascending and the atherosclerotic abdominal aorta is present.
This patient has an aneurysm (defined as having an aortic diameter of greater than 50% larger than normal), with the normal thoracic descending aortic diameter being 2.5 to 3.0 cm. The aortic diameter is frequently assessed to determine the risk for rupture or dissection and also important when describing indications for intervention.
Although the patient is female in this particular case, TAA is two to four times more prevalent in males. Patients with a large TAA have approximately a 25% probability of coexisting abdominal aortic aneurysm and a higher probability of other aortic/arterial pathologies, suggesting that these patients should undergo a CT of the total aorta.
The patient was encouraged to quit smoking immediately as this increases her chance for rupture. Further questioning reveals a negative family history for TAA, Marfan’s syndrome or other connective tissue diseases, and aortic dissection. Serum laboratory studies include hemoglobin of 15 g/dL, normal platelet count, and creatinine of 0.7 mg/dL.
Diagnosis and Treatment
TAA repair is indicated in this patient. In general, recent guidelines suggest that a TAA greater than 5.5 to 6.0 cm or a TAA greater than 5.0 to 5.5 cm with a positive family history for TAA, Marfan’s syndrome, or aortic dissection should be repaired. This 67-year-old female with a large aneurysm of the distal arch and total descending thoracic aorta has met the requirements for thoracic aortic repair with replacement of the descending thoracic aorta, with a maximum diameter of 6.5 to 7.4 cm. Therefore, the options for open TAA repair as well as thoracic endovascular aortic repair (TEVAR) were discussed with the patient and family.
A number of tests were performed to be certain that she is an appropriate candidate for one or both of these options. These tests include a right and left heart catheterization, transesophageal echocardiography (TEE), carotid duplex scanning, pulmonary function testing 6 weeks following her smoking cessation, and ankle-brachial indices. All test outcomes are unremarkable with the exception of identification of mild COPD (FEV1 60%, DLCO 65%) and are adequate enough to proceed with either open or endovascular TAA repair.
In this particular case, an open approach was performed because it became quite apparent that in order to perform TEVAR, she would also require exposure of her infrarenal aorta to deliver the stent graft, as well as the coverage of the left subclavian artery. In our institution for these latter circumstances, we routinely revascularize the left subclavian artery by extra-anatomic bypass from the left carotid artery in order to provide potential persistent collateral blood flow to the spinal cord and the posterior circle of Willis. Given this extensive associated need for other procedures, it was felt that at her relative young age of 67, an open operation may be a more suitable choice. After discussion and consideration of the treatment options with the patient and her family, the patient prefers to be treated with open TAA repair.
Surgical Approach
In case more than the proximal third is resected, adjunctive cerebrospinal fluid drainage via placement of a lumbar drain is accomplished just after induction of anesthesia. In this particular case, hypothermic circulatory arrest was also identified as an important adjunct given the extent of the TAA into the distal arch as well as the need to resect the total descending aorta (i.e., additional neuroprotective strategy).
The standard operative approach for isolated TAA is as follows. The patient is placed in the right lateral decubitus position with the left chest up and is widely prepped and draped. A standard left posterolateral thoracotomy is performed. The left femoral vessels are cannulated via a transverse infrainguinal incision, and the thoracic descending aorta is mobilized for planned resection.
After withdrawal of 2 to 3 units of autologous blood for later reinfusion, the patient is then systemically heparinized and under TEE guidance, a venous cannula is inserted via the left common femoral vein into the right atrium. The left femoral artery is isolated via a transverse arteriotomy, and an 8-mm Dacron graft is sewn in an end-to-side fashion for arterial return. The patient is placed on cardiopulmonary bypass, and the patient is then actively cooled to circulatory arrest to eventually reach a temperature of 18°C. During cooling, the intercostal vessels, which will not be preserved, are externally ligated and divided down. When the patient reaches 18°C, systemic potassium is administered to arrest the heart. Cardiopulmonary bypass is then discontinued, and the patient is drained of volume. A cross clamp is applied at the level of T4 with the flow reduced to half from the femoral artery to keep the lower body perfused. The proximal incision in the descending thoracic aorta is performed, and the proximal anastomosis is created using external felt reinforcement with a running 4-0 polypropylene suture, which is constructed to a Dacron graft with a single prefabricated side branch. Following completion of this anastomosis, the prefabricated side branch is cannulated for cardiopulmonary bypass and flow is then reinstituted to the upper body. The aorta is de-aired out through the open distal end of the graft, and the proximal clamps are placed distal to the prefabricated side branch to restore flow to the upper body and heart.
Attention is then focused on the distal thoracic aorta, where the aorta is mobilized and circumferentially encircled. The distal clamp is placed beyond the location of the intended anastomosis. Flow is maintained to the lower body via the left femoral artery. Active rewarming to normothermia is initiated, and the descending TAA specimen is removed. The Dacron graft is sized to an appropriate length, and the distal anastomosis to the distal descending thoracic aorta is constructed, again using external felt reinforcement. The clamps are released with adequate de-airing maneuvers, and the patient is then separated from cardiopulmonary bypass when normothermic. The patient is decannulated during rewarming from the left groin, and the left femoral artery is then primarily repaired. Protamine sulfate and autologous blood are administered to reverse the coagulopathy. At this point, hemostasis is assured in the aneurysm bed as well as at the anastomoses. Chest tubes are placed. The main thoracotomy and left groin wounds are closed routinely. The wound is dressed at this point, and the patient can be transported back to the intensive care unit (Table 1).
TABLE 1. Open Descending ThoracicAortic Aneurysm Repair




