The investigators describe 35-year-old monozygotic twins who presented 6 months apart with heart failure. In conclusion, this is the first report of adult monozygotic twins with isolated left ventricular noncompaction who presented with similar clinical and echocardiographic features and abnormal twist mechanics.
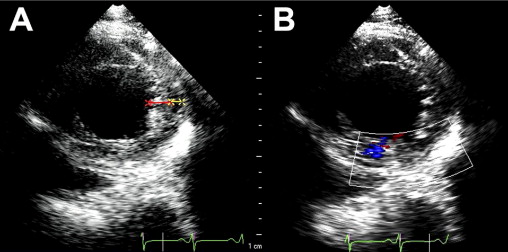
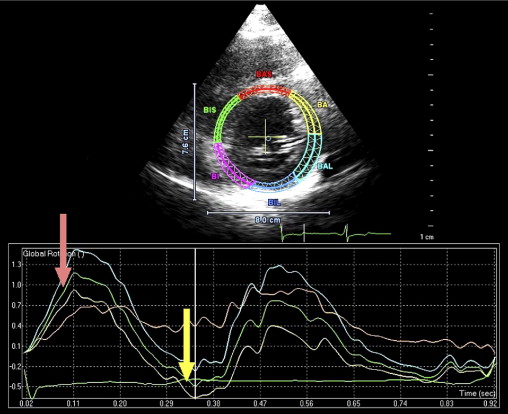
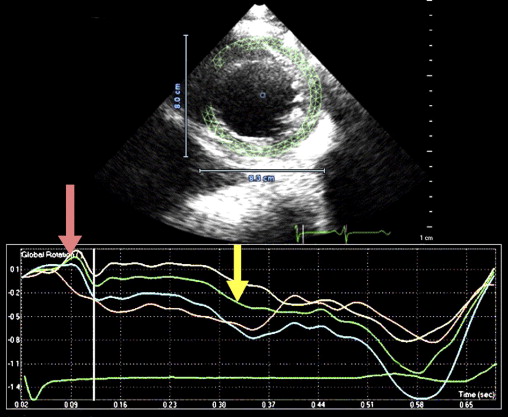
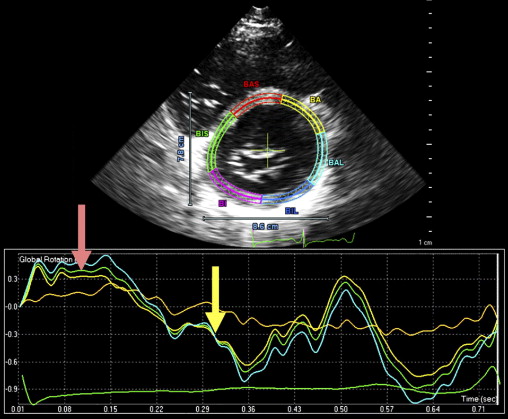
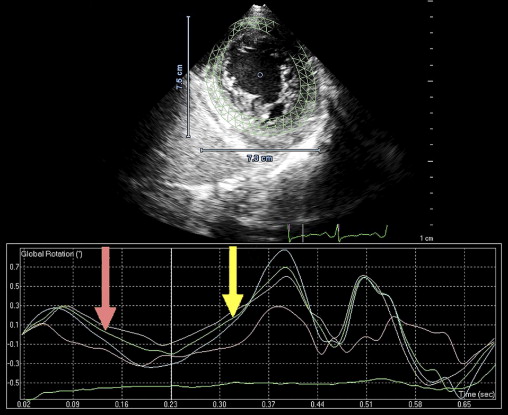
Isolated left ventricular (LV) noncompaction (ILVNC) is presumed to be a genetic cardiomyopathy with familial and sporadic forms. This report describes the case presentations of 35-year-old monozygotic twins who presented 6 months apart with heart failure. In each instance, there was no significant medical history before the onset of symptoms. There were no features suggesting an underlying systemic illness or other predisposition for heart failure. The family history was unremarkable. In each twin, echocardiography confirmed a dilated left ventricle, with a global ejection fraction of 18% in 1 man and 20% in the other. In the 2 cases, the Jenni and Stöllberger criteria for ILVNC were satisfied, with noncompaction established in the apex and midlateral wall ( Figures 1 and 2 , Videos 1 and 2 ). Speckle tracking of the apex and base ( Figures 3 to 6 ) revealed that each twin had clockwise rigid body rotation (RBR), i.e. the apex and base rotated in a clockwise direction during systole.
Case Descriptions
A 35-year-old black man was diagnosed and treated for new-onset heart failure 6 weeks before being evaluated at a tertiary academic cardiomyopathy clinic. He was categorized in New York Heart Association functional class I and was receiving heart failure therapy, which included a loop diuretic, carvedilol, an angiotensin-converting enzyme inhibitor, and spironolactone. His cardiac history before the recent admission was unremarkable, with no preceding viral or systemic illnesses. In addition, there was no history to suggest alcohol or illicit drug abuse. The results of all blood tests to evaluate secondary causes of dilated cardiomyopathy were normal, including human immunodeficiency virus serology. Electrocardiographic findings at rest were normal, except for evidence of a fragmented QRS pattern in the lateral leads. Transthoracic echocardiography revealed an LV end-diastolic diameter of 67 mm and a global ejection fraction of 18%. Doppler echocardiography revealed a restrictive mitral inflow pattern. Mild functional mitral regurgitation was present. The right ventricle was not enlarged but was dysfunctional (tricuspid peak systolic velocity of the mitral annular longitudinal movement [S′] 8.9 cm/s), with no tricuspid regurgitation. Evidence of noncompaction was noted in the apex and midlateral wall, which satisfied the Jenni and Stöllberger criteria ( Figure 1 , Videos 1 and 2 ). Speckle-tracking echocardiography revealed that both the base and apex rotated in a clockwise direction during the ejection phase of systole, providing no systolic twist, a phenomenon termed “rigid body rotation” by van Dalen et al ( Figures 3 and 4 ).
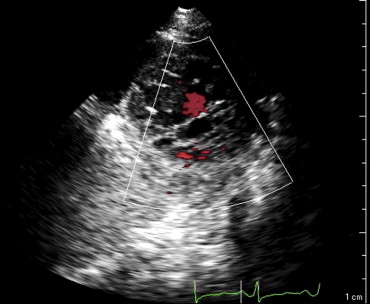
This patient’s twin brother presented with new-onset congestive heart failure approximately 6 months later. The twin was evaluated at the tertiary cardiomyopathy clinic 3 weeks after being categorized in New York Heart Association class II and placed on heart failure therapy (a loop diuretic, carvedilol, an angiotensin-converting enzyme inhibitor, and spironolactone). His cardiac history before the recent admission was unremarkable, with no preceding viral or systemic illnesses. In addition, there was no history to suggest alcohol or illicit drug abuse. The results of all blood tests to evaluate secondary causes of dilated cardiomyopathy were normal, including human immunodeficiency virus serology. Electrocardiography at rest revealed nonspecific T-wave inversion in the lateral leads. Transthoracic echocardiography revealed an LV end-diastolic diameter of 62 mm with a global ejection fraction of 20%. Doppler echocardiography revealed pseudonormalization of the mitral inflow pattern. Mild functional mitral regurgitation was present. The right ventricle was dilated and dysfunctional (tricuspid S′ 6.08 cm/s), with moderate tricuspid regurgitation. Pulmonary systolic pressure was 50 mm Hg. Evidence of noncompaction was noted in the apex and midlateral wall ( Figure 2 , Video 3 ). Speckle-tracking echocardiography at the base and apex revealed clockwise RBR during systole, similar to his twin brother ( Figures 5 and 6 ).
Neither man had any clinical neurologic abnormalities or dysmorphic features. Both men had normal intellectual development. We concluded that a genetic form of ILVNC was likely in these monozygotic twins, who were phenotypically identical.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


