Isolated Iliac Artery Aneurysm
CALOGERO DIMAGGIO, EVAN J. RYER, and JAMES R. ELMORE
Presentation
A 76-year-old male presents to his primary care physician for his routine annual physical examination. He has been in his usual state of health but reports mild right lower abdominal and groin discomfort for the last 3 weeks, which he attributes to overexertion. He denies any recent weight loss, change in bowel habits, dysuria, urgency, or frequency. His medical history is significant for ongoing tobacco use, coronary artery disease, hypertension, chronic kidney disease, and dyslipidemia. His medications include aspirin, simvastatin, hydrochlorothiazide, and carvedilol. On physical examination, blood pressure is 163/95 mm Hg and heart rate is 82 bpm. His abdominal examination reveals no palpable masses or surgical scars. His femoral pulses are 2+, and he has 1+ dorsalis pedis and posterior tibialis pulses bilaterally.
Differential Diagnosis
The differential diagnosis for right lower quadrant and groin discomfort is quite broad. A palpable pulsatile abdominal mass on exam is classic for an abdominal aortic aneurysm (AAA). In an obese patient, an abdominal aneurysm may not be felt, and in a very thin patient, one is expected to palpate a normal-sized aorta. Physical examination is unreliable when it comes to detecting isolated common iliac aneurysms due to their location deep in the pelvis. Computed tomography (CT) scans are the imaging modality of choice for intra-abdominal processes, including aneurysmal disease. Duplex ultrasound screening of the lower extremities should be performed to rule out any peripheral artery aneurysm, particularly femoral and popliteal artery aneurysms, especially in the setting of prominent peripheral pulses.
Workup
As part of his outpatient workup, a CT scan of the abdomen and pelvis was obtained that revealed an isolated 5.2-cm right common iliac artery (CIA) aneurysm (Fig. 1). No evidence of AAA or other gross intra-abdominal pathology was noted (not shown). Serum laboratory studies revealed a hemoglobin level of 13 g/dL, a normal platelet count, a normal prothrombin time, and a serum creatinine of 1.5 mg/dL (glomerular filtration rate [GFR] 47 mL/min).
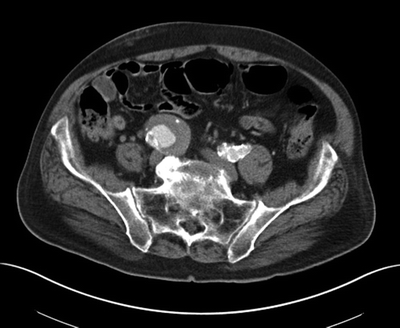
FIGURE 1 CT scan of the abdomen and pelvis with intravenous contrast: 5.2-cm right common IAA. No evidence of AAA or left iliac aneurysm (not shown).
Diagnosis and Treatment
An iliac artery aneurysm (IAA) is any permanent, localized dilation of the iliac artery measuring larger than 1.5 cm in maximal diameter. Many others consider an aneurysm to be a localized expansion of an artery, iliac included, greater than 50% of the normal caliber of the artery. Multiple reports have demonstrated that IAAs most commonly occur in the presence of AAAs. Alternatively, isolated iliac artery aneurysms (iIAAs) are rare and account for less than 3% of all intra-abdominal aneurysms. In approximately 70% of iIAAs, the CIA is involved, followed by the internal iliac artery in 25% of cases. Rarely, isolated external IAAs are encountered. Like AAA, iIAAs are more common in men (5 to 16× more common depending on the series) with most patients presenting after age 65. The natural history of iIAAs, similar to AAA, is one of continued expansion with the potential for life-threatening rupture. In fact, approximately 30% of patients with iIAAs present with rupture accounting for mortality rates as high as 60%, whereas the mortality for elective repair is less than 5%.
Although the clinical presentation of iIAAs is variable, most patients are asymptomatic and are diagnosed after an incidental discovery on imaging studies for unrelated indications. In symptomatic iIAAs, the size and location often dictate the clinical presentation. Very large aneurysms (greater than 5 cm) detected late are more likely to be associated with local compressive symptoms or rupture resulting in hemodynamic collapse. Compression of surrounding structures may include the ureters resulting in hydronephrosis, nerve compression leading to neurogenic pain, bowel compression resulting in intestinal obstruction, or iliac vein compression leading to edema or a venous thromboembolic event. As previously mentioned, the natural history of iIAAs is one of expansion over time with the rate of expansion directly related to iliac artery diameter on initial diagnosis. iIAAs less than 3 cm have demonstrated expansion rates of approximately 1 mm/year, whereas aneurysms greater than 3 cm are estimated to expand by approximately 2.5 mm/year. The average size of ruptured isolated IAAs is between 5 and 7 cm. With the high mortality associated with rupture, current recommendations favor intervening when iIAAs reach 3.5 cm. For iIAAs less than 3 cm, annual duplex ultrasonography is recommended. For those aneurysms greater than 3 cm, but less than the 3.5 cm threshold, serial imaging at 6 months or elective repair for carefully selected cases such as those with continued aneurysm expansion is recommended.
Surgical Approach for Endovascular Iliac Aneurysm Repair
A thorough understanding of the aneurysm’s anatomic characteristics is paramount when planning repair and is achieved only by meticulous review of fine-cut (1-mm) CT scans with sagittal and coronal reconstructions. As in endovascular AAA repair, adequate proximal and distal landing seal zones are critical. Although no formal criteria for adequate neck length exist, a minimum of 1.0 cm length has been recommended for iliac arteries. In addition to adequate neck length, the aneurysm’s anatomic location also factors into the surgeon’s decision making. Unilateral repair is appropriate in individuals with a normal caliber (nonaneurysmal) proximal CIA, but implantation of a bifurcated aortic stent graft is necessary when the proximal CIA is aneurysmal. Aneurysms that extend to the common iliac bifurcation often require internal iliac artery coil embolization with coils or an excluding plug (i.e., Amplatzer plug [AGA Medical Corp, Plymouth, MN]) to prevent retrograde perfusion and a potential type 2 endoleak.
Preparation of the patient for endovascular repair of IAAs is done in a similar fashion to endovascular AAA repair. The procedure can be performed under general, regional, or local anesthesia with sedation if necessary. With the patient supine in the hybrid operating room suite, percutaneous access is obtained using a micropuncture needle. Using the standard Seldinger technique, a guidewire is passed under fluoroscopic guidance. After the appropriate-sized sheath is placed, a marker catheter is passed over the wire and an abdominal/pelvic aortogram obtained. Systemic anticoagulation is next administered. Coil embolization of the internal iliac artery is performed with standard coils or plugs delivered to the origin of the internal iliac artery (Fig. 2). The proximal and distal extent of the CIA aneurysm is identified, and measurements are obtained. Next, the appropriate stent is selected, opened, and prepared for use. Under fluoroscopy, the stent graft device is delivered over the wire to planned graft deployment site. Of note, with regard to stent sizing, a 10% to 20% CIA and 10% external iliac artery oversizing is recommended. Placement is confirmed with retrograde sheath angiogram. Postdeployment stent graft dilation performed to ensure optimal apposition of vessel wall and graft. Finally, perform completion angiogram to assess for endoleak(s).
Recent advances have led to the development of branched iliac artery stent grafts. This technology allows the surgeon to extend a conventional endovascular stent graft repair (i.e., endovascular aneurysm repair [EVAR]) into the external iliac artery while preserving flow into the ipsilateral internal iliac artery. Current literature provides little information about the safety and efficacy of these devices. Additionally, these devices are currently limited to use within clinical trials within the United States. As experience grows, these devices may alter current treatment algorithms. Chimney grafts were intentionally omitted from this chapter as the authors believe this technique is only suitable for very select cases who are not candidates for traditional open or endovascular repair. In the future, it is anticipated that the branched iliac stent grafts will replace the chimney grafts for this iliac aneurysmal disease (Table 1).
TABLE 1. Endovascular Iliac Artery Aneurysm Repair




