When stenting an ostial or proximal coronary lesion, 1 fundamental decision is whether to extend the proximal end of the stent into the aorta (in the case of the left main [LM] or right coronary ostium) or into the polygon of confluence of the LM (in the case of the left anterior descending [LAD] ostium). Complete angiographic and intravascular ultrasound data and 9-month follow-up angiographic and clinical data were available from 459 patients with 138 ostial lesions (angiographic diameter stenosis within the ostium of ≥50%) or 321 nonostial lesions in which the proximal end of the stent ended at or near the coronary ostium. Strut protrusion was more frequent in the LM than in the right or LAD ostium (68% vs 59% vs 53%, p = 0.010). The length of strut protrusion was 3.4 ± 1.7 mm in the LM ostium, 1.7 ± 1.0 mm in the LAD ostium, and 2.4 ± 1.4 mm in the right ostium (p = 0.001). In contrast, incomplete stent coverage of the ostium was similar among the LM, LAD, and right coronary artery (23% vs 33% vs 28%, p = 0.084) with a residual uncovered segment plaque burden of 42 ± 11%. Ostial restenosis was similar between the lesions with versus without strut protrusion (3.2% vs 2.3%, p = 0.775) and between the lesions with incomplete versus complete stent coverage of the ostium (2.4% vs 3.0%, p = 0.100). Ostial restenosis was seen in only 2 of 61 lesions (3.3%) with acute malapposition. In conclusion, when treating an ostial or proximal coronary artery lesion with a drug-eluting stent, the decision of whether to protrude the proximal end of the stent or leave the ostium uncovered does not appear to be critical.
When stenting an ostial or proximal coronary artery lesion, 1 fundamental decision is whether to extend the proximal end of the stent into the aorta (in the case of the left main [LM] coronary artery or right coronary artery) or into the polygon of confluence of the LM (in the case of the left anterior descending [LAD]). In part because of the radiolucency of the stent, this is difficult to assess angiographically. In contrast, intravascular ultrasound (IVUS) can accurately assess the relation between the proximal end of the stent and the true coronary ostium and provide information regarding expansion and stent–vessel wall apposition that is often incomplete in ostial lesions because of the size of the proximal vessel lumen. Thus, the aim of the present study was to use IVUS to assess the relation between the proximal end of a drug-eluting stent (DES) and the coronary ostium to determine whether protrusion, incomplete proximal vessel coverage, or acute malapposition affects subsequent restenosis or major adverse coronary events.
Methods
From March 2008 to September 2010, 493 patients (493 lesions) underwent IVUS-guided DES placement in which the proximal end of the stent was positioned at or near the coronary ostium and in whom 9-month angiography was performed at the Asan Medical Center (Seoul, Korea). The patients were excluded if stent implantation was performed during cardiogenic shock or as a bridge to emergency bypass surgery, if antiplatelet agents were contraindicated, or if the left ventricular ejection fraction was <35%. The lesion-related exclusion criteria were chronic total occlusion, in-stent restenosis, and saphenous vein graft. Because of incomplete IVUS visualization of the ostial segment either before or after stenting, an additional 34 patients were excluded. Thus, a total of 459 lesions (229 LM, 162 LAD, and 68 right coronary ostia) were finally included. All had post-stenting IVUS scans available. After excluding the lesions with predilation before IVUS, preprocedural IVUS scans were available for 354 lesions.
Major adverse coronary events was defined as death from cardiac causes, target lesion revascularization, or myocardial infarction. Revascularization was defined as “ischemia driven” if the angiographic diameter stenosis was ≥50%, with a documented positive functional study such as a thallium scan or treadmill test, ischemic changes on the electrocardiogram, or ischemic symptoms. In addition, lesions with an angiographic diameter stenosis of ≥70% were considered to be “ischemia driven,” even in the absence of documented ischemia. Myocardial infarction was diagnosed by the presence of ischemic symptoms or signs plus cardiac enzyme elevation (creatine kinase-MB elevation >3 times or creatine kinase elevation >2 times the upper limit of normal or troponin I >1.5 ng/ml). The diagnosis of stent thrombosis was according to the Academic Research Consortium criteria. All patients provided written informed consent.
Quantitative angiographic analysis was done using automated edge-detection algorithms (CAAS-5, Pie Medical Imaging, Maastricht, The Netherlands) in the angiographic analysis center of the CardioVascular Research Foundation (Seoul, Korea). All images were independently analyzed by investigators who were unaware of the clinical data. The minimum lumen diameter and diameter stenosis were measured in stent and in segment to include 5-mm-long segments adjacent to the distal stent edge. Aorto-ostial lesions of the LM or right coronary artery were located within 3 mm of the aorta on the least foreshortened angiographic projection. The ostial LAD lesions were within 3 mm distal to the carina. Angiographic restenosis was defined as diameter stenosis of ≥50% at the follow-up examination, and ostial restenosis was defined as <3 mm of the coronary ostium. Patterns of restenosis were assessed using the Mehran classification.
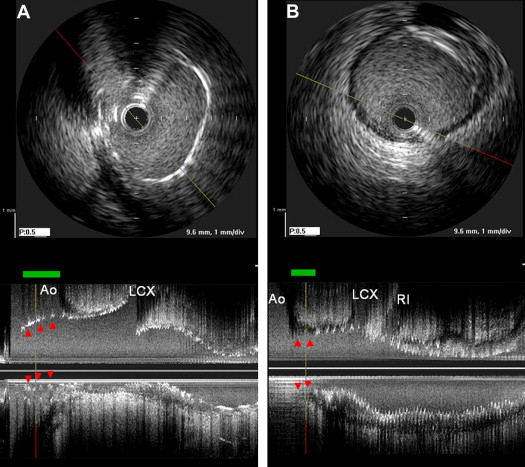
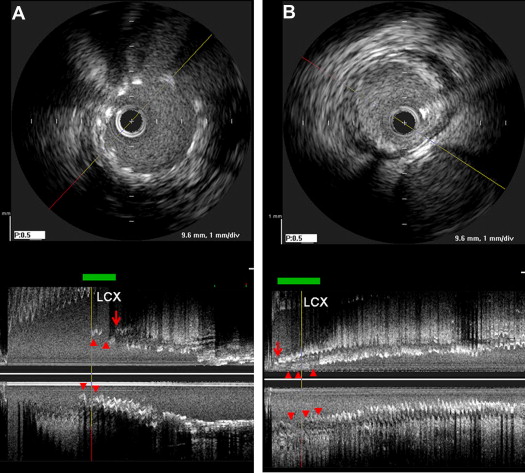
IVUS imaging was performed after intracoronary administration of 0.2 mg nitroglycerin using motorized transducer pullback (0.5 mm/s) and a commercial scanner (Boston Scientific Scimed, Minneapolis, Minnesota) consisting of a rotating 40-MHz transducer within a 3.2F imaging sheath. Using computerized planimetry (EchoPlaque, version 3.0, Indec Systems, Mountain View, California), off-line IVUS analysis was performed. In-stent segment analysis included the minimum lumen area, minimum stent area, and external elastic membrane area. The plaque burden was calculated as follows: ([external elastic membrane − lumen]/external elastic membrane) × 100 (%). Stent underexpansion was defined as <8.0 mm 2 for the LM and <6.0 mm 2 for the LAD and right coronary arteries. The IVUS definition of each ostium paralleled the angiographic definition. In the LM and right coronary ostia, the length of the stent struts protruding into the aorta was measured ( Figure 1 ). In contrast, if full lesion coverage was not present, the length of the ostial segment without stent coverage was also measured ( Figure 1 ). Similarly, in ostial LAD lesions, the length of stent protrusion into the polygon of confluence of the distal LM ostium (distance from the carina to most proximal stent strut) and the length of the uncovered ostium were measured ( Figure 2 ). Malapposition was defined as separation of ≥1 stent strut not in contact with the intimal surface of the vessel wall that was not overlapping a side branch and had evidence of blood speckling behind the strut.
All statistical analyses were performed using SPSS, version 10.0 (SPSS, Chicago, Illinois). All values are expressed as the mean ± SD for continuous variables or as counts and percentages for categorical variables. Continuous variables were compared using the unpaired t test, and categorical variables using chi-square statistics or Fisher’s exact test. p Values <0.05 were considered statistically significant. In the post hoc analysis, parameters were compared among the 3 groups—LM, LAD, and right ostia. Bonferroni corrections were made for multiple comparisons of the continuous variables. All p values were 2-sided, and p >0.05 after Bonferroni correction was considered statistically significant.
Results
The clinical characteristics are listed in Table 1 . Quantitative coronary angiographic data are listed in Table 2 . Overall, 138 lesions were located at the coronary ostium (minimum lumen diameter located at the true ostium with angiographic diameter stenosis ≥50%), and 321 lesions were nonostial (ostial diameter stenosis <50%) but with the proximal end of the stent ending at or near the ostium. The pre- and post-stenting IVUS data are summarized in Tables 3 and 4 .
| Characteristic | Value |
|---|---|
| Age (yrs) | 61.8 ± 9.5 |
| Men | 357 (78%) |
| Smoker | 249 (54%) |
| Hypertension ∗ | 268 (58%) |
| Hyperlipidemia † | 317 (69%) |
| Diabetes mellitus | 173 (38%) |
| Ejection fraction (%) | 58.8 ± 6.4 |
| Acute coronary syndrome | 126 (27%) |
| Previous coronary bypass | 12 (3%) |
| Previous myocardial infarction | 16 (4%) |
| Renal failure ‡ | 36 (8%) |
| Drug-eluting stent type | |
| Endeavor | 52 (11%) |
| Endeavor Resolute | 91 (20%) |
| Promus | 76 (17%) |
| Xience | 160 (35%) |
| Cypher | 77 (17%) |
| Maximal balloon pressure (atm) | 18.8 ± 4.5 |
| Total stent length (mm) | 39.5 ± 18.5 |
∗ Systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg or receiving antihypertensive treatment.
† Total cholesterol >200 mg/dl or receiving antilipidemic treatment.
| LMCA | LAD | RCA | p Value | |
|---|---|---|---|---|
| Lesions (n) | 229 | 162 | 68 | — |
| Preprocedure angiographic data | ||||
| Minimum lumen diameter (mm) | 1.5 ± 0.7 ∗,† | 1.1 ± 0.9 | 1.1 ± 0.6 | <0.001 |
| Diameter stenosis (%) | 57.9 ± 16.4 ∗,† | 68.7 ± 14.1 | 70.4 ± 16.3 | <0.001 |
| Lesion length (mm) | 30.5 ± 16.4 | 34.1 ± 14.5 | 30.9 ± 18.4 | 0.092 |
| Thrombolysis In Myocardial Infarction flow 3 | 197 (87%) | 138 (86%) | 55 (81%) | |
| Post-stenting angiographic data | ||||
| Minimum lumen diameter (mm) | 3.2 ± 0.6 ∗ | 2.7 ± 0.4 | 3.1 ± 0.4 | <0.001 |
| In-stent diameter stenosis (%) | 6.0 ± 8.4 † | 7.2 ± 7.3 | 8.7 ± 6.7 | 0.031 |
| Thrombolysis In Myocardial Infarction flow 3 | 229 (100%) | 162 (100%) | 68 (100%) | 1.000 |
| Follow-up angiographic data | ||||
| Minimum lumen diameter (mm) | 2.9 ± 0.7 ∗,† | 2.5 ± 0.5 | 2.6 ± 0.7 | <0.001 |
| In-stent diameter stenosis (%) | 14.3 ± 15.2 † | 17.0 ± 13.4 ‡ | 26.1 ± 19.1 | <0.001 |
| Angiographic in-stent restenosis | 10 (4.4%) | 6 (3.7%) | 8 (11.8%) | <0.001 |
| Marginal | 2 (20%) | 3 (50%) | 4 (50%) | 0.532 |
| Focal body | 4 (40%) | 2 (33%) | 4 (50%) | |
| Diffuse in-stent | 2 (20%) | 1 (17%) | 0 (0%) | |
| Total occlusion | 2 (20%) | 0 (0%) | 0 (0%) |
∗ p <0.05, LM coronary artery versus LAD.
† p <0.05, LM coronary artery versus right coronary artery.
| Coronary Artery | p Value | |||
|---|---|---|---|---|
| LM | LAD | Right | ||
| Preprocedural intravascular ultrasound of ostial segment | 199 | 117 | 38 | |
| Lumen area (mm 2 ) | 8.6 ± 4.5 ∗ † | 5.8 ± 2.9 | 5.3 ± 2.5 | <0.001 |
| External elastic membrane area (mm 2 ) | 21.3 ± 5.9 ∗ † | 15.8 ± 4.1 | 15.5 ± 4.7 | <0.001 |
| Plaque burden (%) | 60.1 ± 15.5 | 63.8 ± 14.6 | 65.1 ± 14.7 | 0.052 |
| Post-stenting intravascular ultrasound | 229 | 162 | 68 | |
| In-segment | ||||
| Minimum stent area (mm 2 ) | 7.8 ± 2.7 ∗ | 5.7 ± 1.7 ‡ | 7.9 ± 2.0 | <0.001 |
| External elastic membrane area at minimum stent area site (mm 2 ) | 16.8 ± 7.6 ∗ | 10.9 ± 4.1 ‡ | 15.9 ± 4.7 | <0.001 |
| Ostial segment | ||||
| Stent area (mm 2 ) | 11.0 ± 2.6 ∗ † | 8.6 ± 1.6 ‡ | 10.2 ± 2.1 | <0.001 |
| External elastic membrane area (mm 2 ) | 23.6 ± 5.0 ∗ † | 17.8 ± 3.4 ‡ | 19.7 ± 4.0 | <0.001 |
| Stent/vessel area ratio | 0.47 ± 0.09 † | 0.49 ± 0.07 | 0.52 ± 0.08 | <0.001 |
| Strut protrusion | 156 (68%) ∗ † | 86 (53%) | 40 (59%) | 0.010 |
| Length of strut protrusion (mm) | 3.4 ± 1.7 ∗ † | 1.7 ± 1.0 | 2.4 ± 1.4 | <0.001 |
| Strut protrusion >2 mm | 123 (54%) ∗ † | 25 (15%) ‡ | 21 (31%) | <0.001 |
| Strut protrusion >3 mm | 89 (39%) ∗ † | 6 (4%) ‡ | 14 (21%) | <0.001 |
| Incomplete ostial stent coverage | 53 (23%) | 54 (33%) | 19 (28%) | 0.084 |
| Uncovered segment length (mm) | −2.3 ± 1.3 | −1.8 ± 1.1 | −1.7 ± 1.0 | 0.050 |
| Uncovered segment >2 mm | 28 (12%) | 22 (14%) | 7 (10%) | 0.782 |
| Plaque burden within uncovered ostial segment (%) | 38.1 ± 11.9 ∗ | 45.1 ± 10.8 | 40.6 ± 8.5 | 0.006 |
| Malapposition ostium | 43 (19%) ∗ † | 10 (6%) | 9 (12%) | 0.001 |
† p <0.05, LM versus right coronary artery.
| Coronary Ostial Lesion | Nonostial Coronary Lesion | |||||
|---|---|---|---|---|---|---|
| LM | LAD | Right | LM | LAD | Right | |
| Preprocedural intravascular ultrasound | 57 | 23 | 15 | 142 | 94 | 23 |
| Lumen area within ostial segment (mm 2 ) | 5.0 ± 2.0 | 3.1 ± 1.6 | 4.6 ± 2.3 | 10.0 ± 4.5 ∗ | 6.4 ± 2.7 ∗ | 5.7 ± 2.5 |
| External elastic membrane area within ostial segment (mm 2 ) | 18.6 ± 5.3 | 14.9 ± 4.5 | 14.3 ± 5.1 | 22.4 ± 5.9 ∗ | 16.0 ± 4.0 | 16.2 ± 4.4 |
| Plaque burden within ostial segment (%) | 71.5 ± 10.9 | 78.9 ± 9.4 | 66.1 ± 15.7 | 55.4 ± 14.6 ∗ | 60.1 ± 13.2 ∗ | 64.4 ± 14.3 |
| Post-stenting intravascular ultrasound | 65 | 43 | 30 | 164 | 119 | 38 |
| Stent area within ostial segment (mm 2 ) | 10.9 ± 2.3 | 8.3 ± 1.6 | 9.7 ± 1.9 | 11.0 ± 2.7 | 8.7 ± 1.6 | 10.6 ± 2.1 |
| External elastic membrane area within ostial segment (mm 2 ) | 22.5 ± 5.2 | 17.5 ± 3.5 | 19.4 ± 4.4 | 24.1 ± 4.8 ∗ | 17.9 ± 3.5 | 20.1 ± 3.7 |
| Lesions with strut protrusion | 61 (94%) | 31 (72%) | 25 (83%) | 95 (58%) ∗ | 55 (46%) ∗ | 15 (40%) ∗ |
| Strut protrusion length (mm) | 3.7 ± 1.7 | 1.7 ± 1.0 | 2.4 ± 1.5 | 3.1 ± 1.7 ∗ | 1.7 ± 1.0 | 2.3 ± 1.3 |
| Strut protrusion length >2 mm | 54 (83%) | 9 (21%) | 12 (40%) | 69 (42%) ∗ | 16 (13%) | 9 (24%) |
| Strut protrusion length >3 mm | 38 (59%) | 3 (7%) | 9 (30%) | 51 (31%) ∗ | 3 (3%) | 5 (13%) |
| Incomplete ostial stent coverage | 1 (2%) | 6 (14%) | 2 (7%) | 52 (32%) ∗ | 48 (40%) ∗ | 17 (45%) ∗ |
| Uncovered segment length (mm) | −0.4 | −1.6 ± 1.0 | −1.6 ± 1.4 | −2.4 ± 1.3 | −1.8 ± 1.1 | −1.7 ± 1.0 |
| Uncovered segment >2 mm | 0 (0%) | 2 (5%) | 1 (3%) | 28 (17%) ∗ | 20 (17%) | 6 (16%) |
| Plaque burden within uncovered ostial segment (%) | 45.8 ± 14.0 | 44.6 ± 14.0 | 41.7 ± 9.5 | 37.8 ± 12.0 | 45.1 ± 10.5 | 40.4 ± 8.5 |
| Malapposition | 9 (14%) | 0 (0%) | 3 (10%) | 34 (21%) | 10 (8%) ∗ | 5 (13%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


