Chapter 23 Although the prevalence of rheumatic mitral valve (MV) disease remains high worldwide, 1 early treatment of rheumatic fever has altered etiologic patterns in industrialized countries, resulting in a higher prevalence of degenerative and ischemic etiologies (see Chapter 1). As patients age, the prevalence of valve disease increases, with a disproportionate representation of those with mitral valve involvement; 3 this change is in part due to the prolonged survival of patients with severe heart failure and ischemic MR. Therefore the population of patients who present for mitral valve surgery has altered considerably over the past few decades. The MV complex consists primarily of the anterior and posterior leaflets but also includes a number of anatomic entities that are in close proximity to the valve, and all components of the valve must act in a coordinated fashion to ensure proper valve function 4 (see Chapter 2). The mitral annulus effectively separates the left atrium (LA) and left ventricle (LV) and provides support for the anterior and posterior leaflets 5 ( Figure 23-1). Anteriorly, the annulus is interrupted by the aortic-mitral fibrous continuity, with thickened tissue at the right and left fibrous trigones. From the trigones emanates the fibrous tissue of the annulus that encircles the orifice of the valve, but it is rarely continuous; it is deficient in some areas and curtain-like in others. The annulus is not a static structure; it changes size during the cardiac cycle to facilitate filling and minimize regurgitation. 6 The annulus is saddle-shaped, with the low points at the commissures and the high points at the mid-portions of the leaflets. This shape facilitates valve closure and may minimize leaflet stress. 7 FIGURE 23-1 Photomicrograph of the posterior mitral annulus. Whereas the echocardiographer readily identifies the annulus as the hinge point at the base of the leaflets, the surgical identification is the level of the visible transition between the LA myocardium and the denser white leaflet ( Figure 23-2). FIGURE 23-2 Surgical exposure of the mitral valve. The mitral leaflets are in fact a single structure that becomes confluent at the lateral and medial commissures. The anterior leaflet, though longer, covers approximately one third of the annular circumference, and the posterior leaflet covers the remaining two thirds. The posterior leaflet has three scallops: P1, or lateral; P2, or central; and P3, or medial, with the central scallop usually the largest. 8 Although the anterior leaflet lacks distinctive scallops, the nomenclature is such that the portions opposite the corresponding posterior segments are named A1, A2, and A3 ( Figure 23-3). 9 FIGURE 23-3 Anatomic view of the cardiac valves from the perspective of the base of the heart with the left and right atria “cut away” and the great vessels transected. Each leaflet consists of a smooth zone and a rough zone. The rough zone is involved in coaptation and is subtended by primary (marginal, first-order) chordae that insert into the leaflet edges, and secondary (basal, second-order) chordae that insert into the ventricular surface of the rough zone ( Figure 23-4). During systole, the rough zones are in contact over a distance of approximately 1 cm. The excess valvular tissue relative to orifice size offers some functional reserve, thus ensuring proper coaptation and preventing regurgitation. FIGURE 23-4 Mitral valve anatomy. Some especially large second-order chordae, known as strut chords, attach to the rough zone of the anterior leaflet and maintain direct continuity among the valve, the papillary muscles, and the ventricular myocardium. Cutting these strut chords during surgical procedures may lead to LV dysfunction.10,11 Tertiary chordae insert into the basal portion of the posterior leaflet only and are of uncertain significance. The remainder of each leaflet is made up of a smooth zone that is devoid of chordae. Finally, LV shape and myocardial function are also key components in normal MV function. Disturbances in LV function or shape, such as chronic myocardial ischemia, may lead to valvular tethering and mitral regurgitation. 12 One of the keys to successful communication between the echocardiographer and the surgeon is make sure they speak the same “language.” The same structure may be named differently depending on the anatomical terms of reference used. 13 For example, the lateral and medial commissures are sometimes referred to as anterior or left and posterior or right commissures, respectively. The anterior leaflet is intimately associated with the aortic mitral curtain and thus is sometimes referred to as the aortic leaflet. The posterior leaflet may be referred to as the mural leaflet, owing to its proximity to the LV wall. The classification endorsed by the American Society of Echocardiography and Society of Cardiovascular Anesthesiologists is illustrated in Figure 23-5. From left to right (or lateral to medial), the posterior leaflet is divided into scallops P1, P2, P3, and corresponding segments of the non-scalloped anterior leaflet into A1, A2, A3. Deviant clefts may be found in up to 30% of posterior leaflet specimens. 14 FIGURE 23-5 Transesophageal echocardiography views of the mitral valve. The variety and acuity of diagnoses seen in patients coming to the operating room for treatment of valve disease has increased considerably as surgical options have expanded over the past few decades. 15 In addition to regurgitant or stenotic lesions, mixed stenosis and regurgitation and “repeat” surgery for prosthetic valve dysfunction or after a prior valve repair procedure are increasingly common. Ideally the echocardiographer and surgeon should discuss the nature of the mitral disease and the planned operative approach, including any ancillary procedures, such as a maze procedure for atrial fibrillation. Remaining uncertainties after preoperative evaluation should be defined, with a plan for their resolution. Knowledge of preoperative data is crucial. Along with clinical data, the preoperative transthoracic echocardiography (TTE) data should be reviewed, and if possible, the actual images should be examined to assess data quality. Cardiac catheterization findings, computed tomography images, and cardiac magnetic resonance imaging data also should be reviewed when available. The preoperative evaluation helps define the information needed from the intraoperative examination. If, as often occurs, previously undiagnosed pathology is discovered on echocardiography, this information should be promptly shared with the surgeon. In some cases, the referring cardiologist may be consulted if findings are substantially different from expected or a major alteration in surgical approach is needed. The baseline TEE examination includes 2D, spectral, and color-flow Doppler with quantitation of mitral stenosis and regurgitation using standard approaches (see Chapter 6). 3D imaging if available, enhances the understanding of abnormal mitral function., 16 Secondary effects on other structures, specifically the left-sided chambers and the tricuspid valve, may help determine the chronicity of the process. A number of lesions are often associated with MV disease, both primarily and secondarily ( Table 23-1), that may require correction at the time of mitral surgery. TABLE 23-1 Perioperative Implications of Lesions Associated with Mitral Valve Disease On 2D imaging, the general condition of the leaflets is assessed, with the degree of thickness, mobility, and calcification, and subvalvular disease noted. 17 The LA is assessed for the presence of thrombus and ruptured chordae. The presence of masses should alert the echocardiographer to the likelihood of endocarditis, with the possibility of para-annular extension, leaflet perforation, and the involvement of other valves (see Chapter 25). Although uncommon, involvement of the mitral-aortic intravalvular fibrosa (MAIVF) with pseudoaneurysm formation may result from primary mitral rather than aortic endocarditis. 18 Next, a systematic examination of the MV is performed using schemata such as described by Shanewise et al 19 and Foster et al 9 ( Figures 23-5 and 23-6, Table 23-2), which provide a “roadmap” for recognizing where the pathologic aspects of the valve lie. Basic views of the MV leaflets are obtained from a high TEE position ( Figure 23-7). Once each view is obtained, slight movements of the probe—withdrawal and advancement, rotation left and right, and flexion and extension—are used to completely examine each leaflet segment. At this stage of the examination, color-flow Doppler imaging may be used, but more to help clarify the mechanism of MR ( Figures 23-8 and 23-9). The subvalvular apparatus is best seen with transgastric views, which allow visualization of cordal thickening, redundancy, or frank rupture along with the orientation of the papillary muscles. On the basis of these images, the Carpentier classification can be used to define the mechanism and etiology of MR, which may be helpful in the planning of the surgical approach 2 ( Table 23-3; Figure 23-10). TABLE 23-2 Recommended Intraoperative Transesophageal Echocardiography (TEE) Examination *Mahmood F, Hess PE, Matyal R, et al. Echocardiographic anatomy of the mitral valve—a critical appraisal of two-dimensional imaging protocols with a three-dimensional perspective. J Cardiothorac Vasc Anesth 2012;26:777–84. FIGURE 23-6 Effect of changes in transesophageal echocardiography probe position. FIGURE 23-7 Basic transesophageal echocardiography views of the MV. FIGURE 23-8 Anteriorly directed mitral regurgitant jet. FIGURE 23-9 Ischemic mitral regurgitation (MR). FIGURE 23-10 Carpentier’s classification of mitral regurgitation (MR) based on leaflet motion. Measurement of annular diameter may help define the etiology of MR and guide the surgeon in selection of a prosthesis or annuloplasty ring. The saddle shape of the annulus is demonstrated with 3D reconstructions (see Figure 2-3). The low points of the “saddle” are at the commissures, seen in the bicommissural view, and the high points in the anterior-posterior axis, seen in the midesophageal long-axis view. On the basis of comparison with cardiac computed tomography, the best approach for annular measurement is the commissure-to-commissure peak systolic diameter in the TEE bicommissural view the and anterior-to-posterior diameter in the long-axis view. 19a The annulus is also assessed for the degree of calcification, which may be predictive of paravalvular leaks 20 and perioperative vascular events. 21 Examination of global and segmental LV function is also needed in evaluation of the mechanism of MR. Secondary MR is due to either global or regional LV systolic dysfunction or to altered LV geometry. However, chronic primary MR also leads to LV dilation with the potential for progressive LV dysfunction 22 (see Chapter 5), which may complicate the perioperative management of MV surgery. If TEE images are suboptimal, the surgeon can employ the technique of epicardial echocardiography both before and after cardiopulmonary bypass. 23 A transthoracic probe is placed inside a sterile sheath, which is then placed directly on the heart. Most standard transthoracic views can be obtained, with excellent resolution. The most important confounding variables in the intraoperative assessment of MR are the loading conditions, which are affected to a great degree by (1) the depressive effects of general anesthetic on myocardial contractility and vascular tone and (2) the effects of positive pressure ventilation on systemic venous return in both open heart and closed chest procedures. 24 For these reasons, the degree of intraoperative MR is often significantly less than seen on preoperative transthoracic studies. 25 Sometimes this finding creates uncertainty as to the proper surgical course of action. A number of strategies have been proposed to replicate findings in the preoperative state. In a prospective study of patients with at least moderate MR from a variety of etiologies, TEE was performed at three stages: with conscious sedation prior to induction of anesthesia, after induction, and after use of phenylephrine to bring the blood pressure back to pre-induction levels. 25a Blood pressure dropped significantly after induction and was driven over baseline with phenylephrine. Compared with pre-induction findings, there were decreases in measurements of vena contracta, regurgitant orifice area (ROA), and regurgitant volume (RV), although the decreases were not statistically significant. Phenylephrine resulted in the return of regurgitant parameters to baseline, with a significant increase in MR severity compared to post-induction values, regardless of the underlying etiology, likely as a result of the combination of increased blood pressure, changes in preload, and possible myocardial ischemia. In another study of patients with ischemic MR, both phenylephrine and fluids were used to restore pre-induction hemodynamics. Again, parameters of MR severity dropped after anesthesia induction, though not significantly. With loading, blood pressure, ROA, and regurgitant volume superseded baseline measurements. 25b However, these effects are not seen uniformly in all patients. In a diverse group of patients with MR, MR severity remained less than baseline values in 20% of patients despite the use of vasoactive agents to bring blood pressure back to baseline. 25c These studies, in combination with clinical experience, emphasize that intraoperative Doppler estimation of MR is complex. Measures of MR severity are affected by the degree of blood pressure drop, changes in preload and afterload, LV contractility, LV dyssynchrony, 26 mitral closing force, 12 the etiology of mitral disease, other concurrent valve lesions, and the possible induction of myocardial ischemia. The use of pharmacologic manipulation to reestablish baseline conditions is to some extent artificial, and the significance of increased MR severity with “overdriving” of loading parameters is uncertain. It is axiomatic that a high-quality preoperative echocardiogram performed without general anesthesia should be readily available for review in the operating room. Decisions based on the patient’s clinical course and symptoms, degree of LV dilation and systolic dysfunction, and quantitation of MR on the preoperative study should rarely be overruled simply on the basis of differences in the quantitative parameters of MR severity on intraoperative TEE.
Intraoperative Echocardiography for Mitral Valve Surgery
Mitral Valve Disease in the Twenty-First Century
Anatomic Background
The Mitral Valve Complex
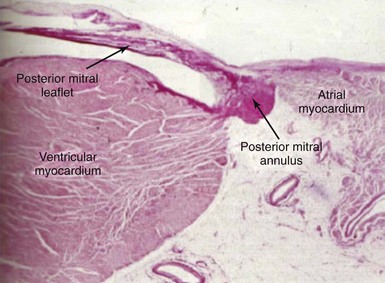
Note that the posterior mitral annulus separates the musculature of the left ventricle from that of the left atrium, where it forms a hinge point with base of the posterior mitral leaflet. (Wilcox, BR, Cook AC, Anderson RH, Surgical anatomy of the valves of the heart. New York: Cambridge University Press; 2004. p. 55, with permission)
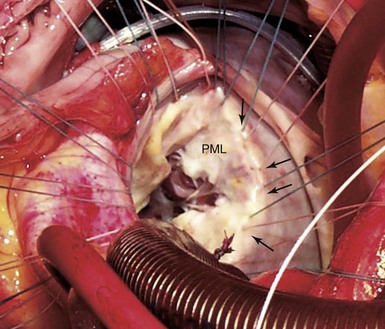
The surgeon has placed sutures in the posterior annulus, which is identified as the transition between the pink atrial myocardium and the white leaflet (arrows). PML, Posterior mitral leaflet.
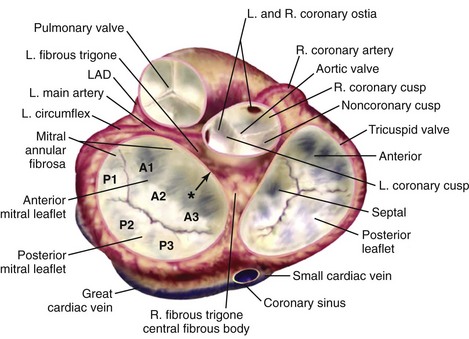
Note the close anatomic relationships of all four cardiac valves. In particular the aortic valve is adjacent to the mitral valve along the midsegment of the anterior mitral valve leaflet. The pulmonic valve is slightly superior to the aortic valve, and the aortic and pulmonic valve planes are nearly perpendicular to each other. The three scallops of the posterior mitral leaflet are lateral (P1), central (P2), and medial (P3) with the corresponding segments for the anterior leaflet (A1, A2, A3) shown. Asterisk indicates the mitral-aortic curtain. L, Left; LAD, left anterior descending artery; R, right.
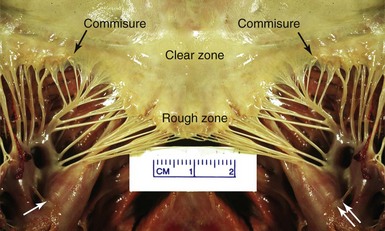
The posterior leaflet has been sectioned at its midpoint. The commissures and the clear and rough zones of the anterior mitral leaflet are shown. The anterolateral (single white arrow) and the posteromedial (double arrow) papillary muscles both give cords to both leaflets. (Image courtesy Dr. Dennis Reichenbach.)
Nomenclature
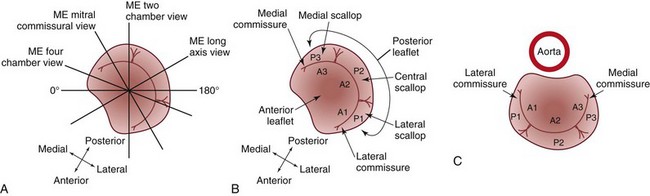
A, Short-axis drawing of the mitral valve illustrating how it is transected by midesophageal views. Rotating through multiplane angles from 0 degrees to 180 degrees moves the imaging plane axially through the entire mitral valve. B, Anatomy of mitral valve. C, The surgeon’s view of the mitral valve. A1, Lateral third of the anterior leaflet; A2, central third of the anterior leaflet; A3, medial third of the anterior leaflet; ME, midesophageal; P1, lateral scallop of the posterior leaflet; P2, central scallop of the posterior leaflet; P3, medial scallop of the posterior leaflet. (From Shanewise JS, Cheung AT, Aronson S, et al. ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography. J Am Soc Echocardiogr 1999;12:884–900.)
Pre-Bypass Assessment
Presurgical Preparation
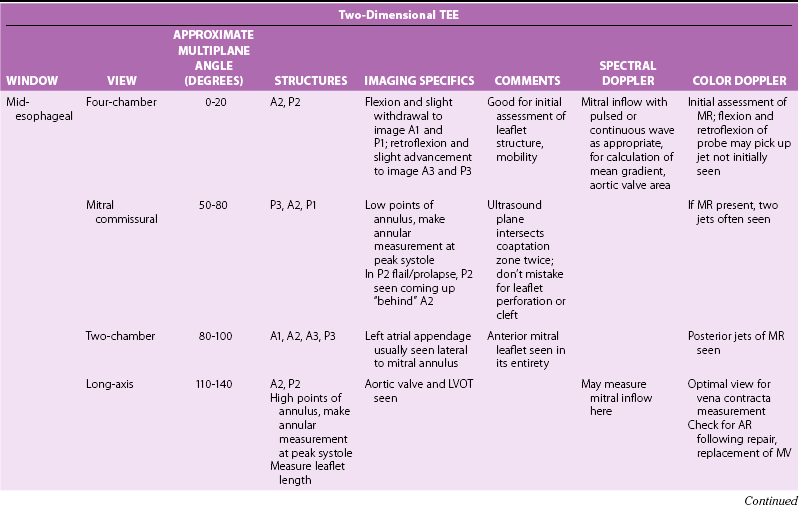
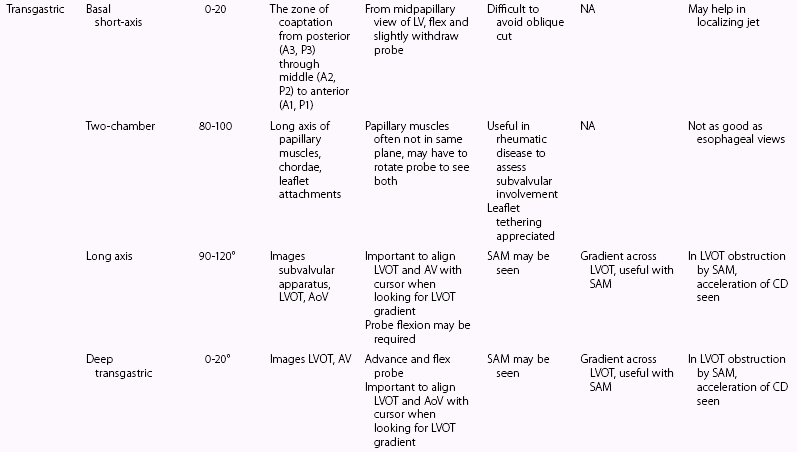
Systematic Examination
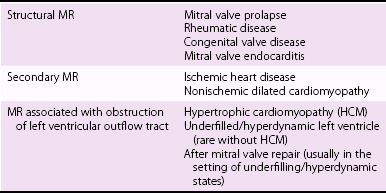
SECONDARY CONDITION
PREOPERATIVE SIGNIFICANCE
POSTOPERATIVE SIGNIFICANCE
Pulmonary hypertension
May indicate left ventricular failure, left ventricular outflow tract obstruction, aortic valve disease, severe mitral regurgitation
Aortic valve surgery may be needed
Pharmacologic therapy may be needed
Right heart failure
Often secondary to elevated left-sided filing pressures
Aggressive pharmacologic support may be needed
Tricuspid regurgitation
Often secondary to elevated left-sided filling pressures, consideration for reparative procedure at time of mitral surgery
Must be differentiated from primary tricuspid valve disease
Mitral leaflet systolic anterior motion
Consideration for reparative procedure; i.e., myomectomy
Aggressive pharmacologic manipulation may be needed; may necessitate valve replacement
Rheumatic valvular disease
May have aortic stenosis, aortic regurgitation, tricuspid stenosis/regurgitation necessitating intervention
Aortic regurgitation may confound mitral valve area calculation by pressure half time method
Reassessment of native valves, or of repaired/replaced valves after mitral valve surgery, required
Two-Dimensional Imaging
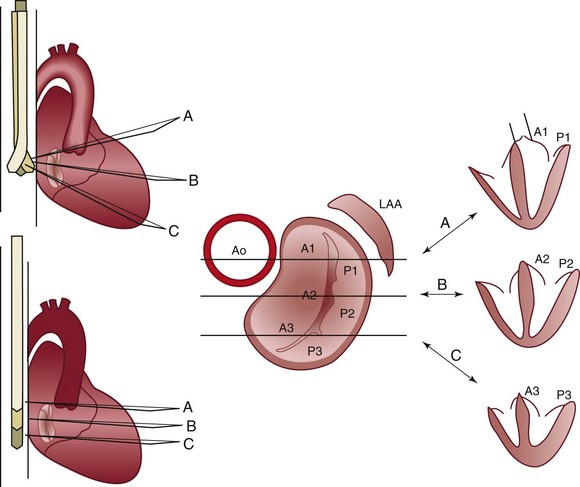
Effect of flexion or withdrawal and retroflexion or advancement of the transesophageal probe tip on the imaging plane in relation to the mitral valve at a transducer rotational angle of 0 degrees. A1, A2, A3, Anterior leaflet sections; Ao, aorta; LAA, left atrial appendage; P1, P2, P3, posterior leaflet sections. (From Foster GP, Isselbacher EM, Rose GA, et al. Accurate localization of mitral regurgitant defects using multiplane transesophageal echocardiography. Ann Thorac Surg 1998;65:1025–31.)
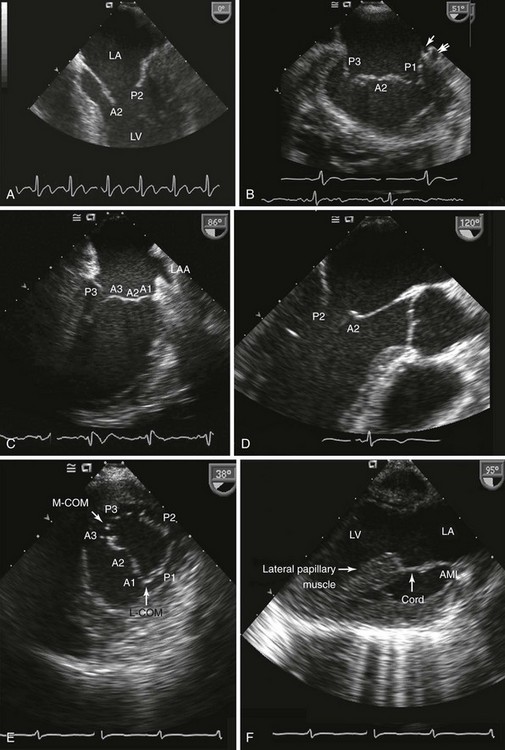
A, Four-chamber view; B, bicommissural view; C, midesophageal two-chamber view; D, midesophageal long-axis view; E, transgastric short-axis view; F, transgastric two-chamber view. A1, A2, A3, Anterior leaflet sections; AML, anterior mitral leaflet; LA, left atrium; L-COM, anterior commissure; LV, left ventricle; M-COM, posterior commissure, P1, P2, P3, posterior leaflet sections.
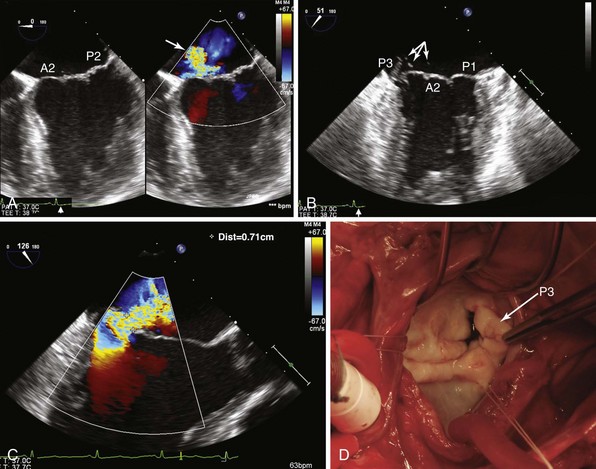
The 46-year-old patient presented with increasing shortness of breath over several years and an acute increase in dyspnea over the last week. A, A four-chamber view (left) demonstrates normal coaptation, but a color-flow Doppler image (right) shows an anteriorly directed jet of mitral regurgitation (MR) (arrow), indicative of either posterior leaflet prolapse or anterior leaflet restriction. B, The bicommissural view shows a flail P3 scallop with numerous ruptured chordae (arrows). C, A vena contracta of 0.71 cm, indicative of severe MR. D, Surgical exposure revealed involvement of the P3 scallop. A2, Anterior section; P1, P2, posterior leaflet sections.
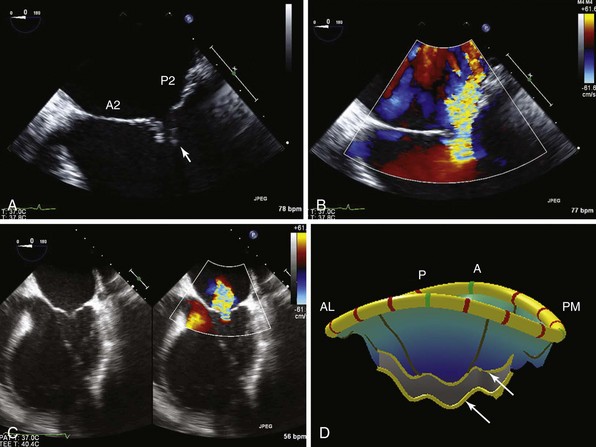
In this patient with previous inferior wall infarction, A demonstrates tethering of the posterior mitral leaflet (arrow) in the four-chamber view. B, Color-flow Doppler image shows a posterior directed jet of MR. C, In another patient with a dilated cardiomyopathy and symmetric tethering, a central jet of MR is shown. D, MVQ (mitral valve quantification) illustrates the bileaflet tethering (arrows). (With permission, Stefan Lombaard) A, anterior; AL, antero-lateral; P, posterior; PM, postero-medial.
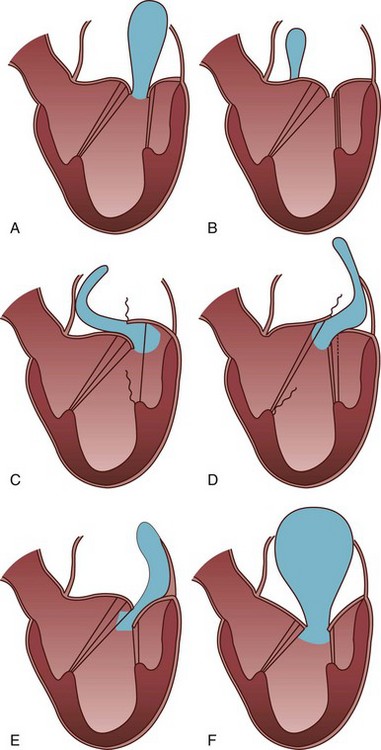
Leaflet motion is classified as normal (Type I), excessive (Type II) or restricted (Type III). In type I (A and B) the leaflet motion is normal and the jet tends to be central. The cause of MR is usually annular dilatation (A) or leaflet perforation (B). In type II (C and D) there is excessive leaflet motion and the jet is directed away from the diseased leaflet. In type III lesions (E and F) the leaflet motion is restricted. Type III lesions are further subdivided into IIIA and IIIB. The jet can be directed towards the affected leaflet, or it can be central if both leaflets are equally affected. (Modified from Perrino AC, Reeves ST. The practice of perioperative transesophageal echocardiography. Philadelphia: Lippincott William & Wilkins; 2003.)
Epicardial Echocardiography
Loading Conditions
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Intraoperative Echocardiography for Mitral Valve Surgery
Only gold members can continue reading. Log In or Register to continue