CCA, common carotid artery; ICA, internal carotid artery.
Intraoperative assessment with duplex scanning also provides a dynamic means to identify and characterize arterial pathology to be repaired. For example, intraoperative duplex scanning can find focal stenoses from extrinsic compression due to popliteal entrapment or median arcuate ligament compression, and then it can be used to verify normal hemodynamics after release of the compressive fibromuscular bands.9–11
Intraprocedural testing using duplex or intravascular ultrasound (IVUS) can be done in less than 10 minutes ultrasound is more convenient than arteriography; and study interpretation is based on straightforward criteria.2–5,8
TECHNIQUES FOR INTRAOPERATIVE ASSESSMENT
Duplex scanning is complementary to other intraoperative assessments such as visual inspection, pulse palpation, continuous-wave (CW) Doppler flow analysis, and angiography. Repair site imaging is typically performed after the intervention is completed but before the incision is closed or catheter access is terminated. At this point in the procedure, inspection and pulse assessment are considered to be normal or acceptable. In this setting, duplex scanning will identify an unsuspected problem in 3% to 15% of cases, with the nature of the problem dependent on the anatomic site of the arterial intervention.1–5 When the initial clinical assessment or a completion angiogram is abnormal or equivocal, duplex testing can be helpful in further defining the residual abnormality based on anatomic and hemodynamic criteria. Application of duplex ultrasound is feasible for all open surgical arterial repairs and transcutaneous imaging of peripheral angioplasty procedures (Table 30.2); however, carotid, iliac, and mesenteric or renal artery angioplasty procedures are better assessed for technical adequacy with angiography, pressure measurements, or IVUS findings.
TABLE 30.2 Diagnostic Methods and “Normal” Threshold Criteria for Intraprocedural Assessments of Open Surgical and Endovascular Peripheral Arterial Repairs
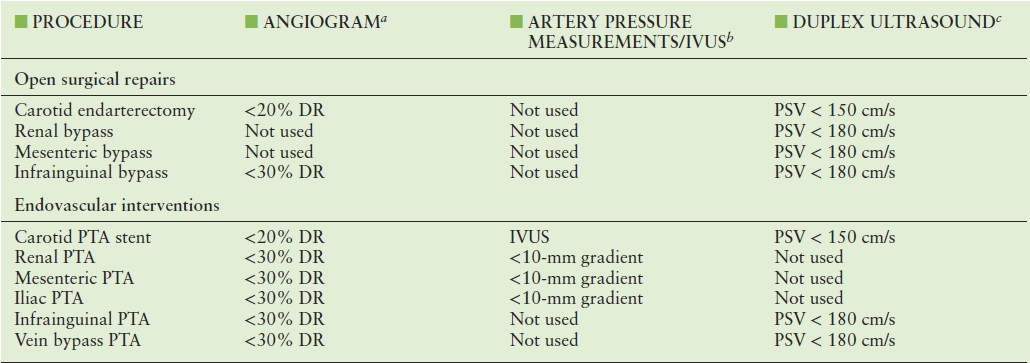
aCriteria for acceptable residual stenosis, expressed as % DR.
bMeasurement of systolic pressure gradient, expressed as mm Hg.
cPSV threshold for revision of residual stenosis, used in conjunction with B-mode imaging criteria of stenosis and PSV ratio of >2.0 at the site of abnormality.
DR, diameter reduction; IVUS, intravascular ultrasound; PSV, peak systolic velocity; PTA, percutaneous transluminal angioplasty.
Transducer Selection
A high-frequency (10 to 15 MHz) hockey stick–shaped, linear array transducer is preferred for detailed intraoperative imaging. Asepsis is maintained by placing the transducer in a sterile plastic sleeve, with gel for acoustic coupling (Fig 30.1). To image deeper vessels (carotid stent, lower limb angioplasty sites, anatomic tunneled bypass grafts), a 5- to 7-MHz linear array transducer is used to scan transcutaneously over the area of interest.
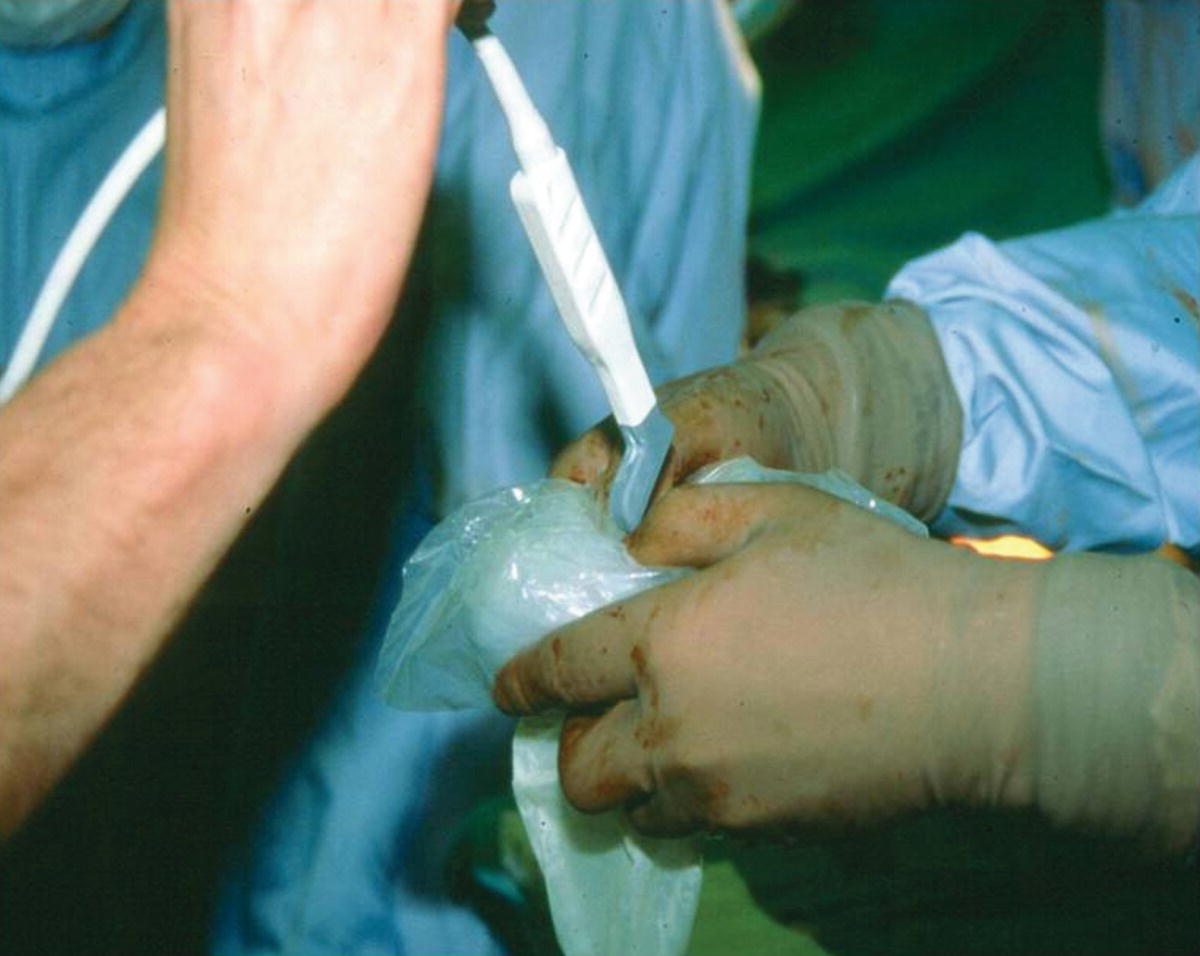
FIGURE 30.1. A 12-MHz “hockey stick” linear array transducer is inserted in a sterile plastic sleeve filled with ultrasound gel for use in the operative field.
Scanning Technique
The assistance of a vascular technologist to optimize instrument settings facilitates testing, whether performed in the operating room or interventional angiography suite. The surgeon manipulates the transducer over the arterial repair, assessing the B-mode image and color Doppler display, and directs where velocity spectra should be recorded, including any identified abnormalities. Sterile saline is instilled into the operative wound for acoustic coupling, and the transducer is positioned over the artery for scanning in sagittal and transverse planes (Fig. 30.2). Pulsed Doppler velocity spectra should be recorded from a longitudinal image of the artery using an ultrasound beam angle of 60 degrees or less to the vessel wall. The entire arterial repair should be imaged to assess lumen caliber and identify major anatomic defects such as residual plaque, stenosis, or wall dissection. Color Doppler imaging is useful to detect and interrogate sites of flow disturbance produced by flaps, kinks, stenosis, and retained vein conduit valves.
The sequence of scanning should begin at the inflow artery of the repair and proceed distally with imaging of the entire reconstruction and outflow artery. For carotid endarterectomy or visceral artery repairs, only a 10 to 15 cm vessel segment needs to be imaged, while evaluation of an infrainguinal vein bypass requires additional time to image the vein conduit, anastomotic regions, and the inflow/outflow arteries. Testing should be performed when the patient’s hemodynamic status is normal. If hypotension is present, the condition should be corrected before scanning commences. If arterial spasm, a high-resistance flow state, or low graft flow velocity is detected, direct intra-arterial injection of papaverine (30 to 60 mg) can augment flow by reducing peripheral vascular resistance, thereby enhancing diagnostic accuracy for stenosis detection. Of note, papaverine should not be injected into the cerebral circulation. (Intra-arterial nitroglycerine 100 to 200 μg can be used to reduce carotid vasospasm.)
Arteriographic imaging is already an integral part of most endovascular procedures, and many surgeons also utilize completion angiography after carotid endarterectomy or lower limb arterial intervention,8,12–16 but duplex scanning is more sensitive for detecting some types of abnormalities.12–14,17–19 Duplex scanning adds hemodynamic assessment of repair sites and the detection of subtle abnormalities such as vein valve stenosis and intimal flaps. The presence and severity of a lesion can be characterized using pulsed Doppler spectral recordings with measurement of peak systolic velocity (PSV) and the velocity ratio (Vr) across the stenosis. The clinical value of duplex testing is based on the rationale that if a residual lesion is identified, additional intervention can be performed immediately with the goal of achieving normal repair site hemodynamics.
The vascular laboratory should allot approximately 30 minutes for intraoperative examinations, including instrument transport, setup, scanning, and image archiving. When intraoperative duplex scanning is routinely employed, most scans will be uncomplicated (i.e., associated with “normal” findings) and will typically require less than 10 minutes of hands-on scanning time.
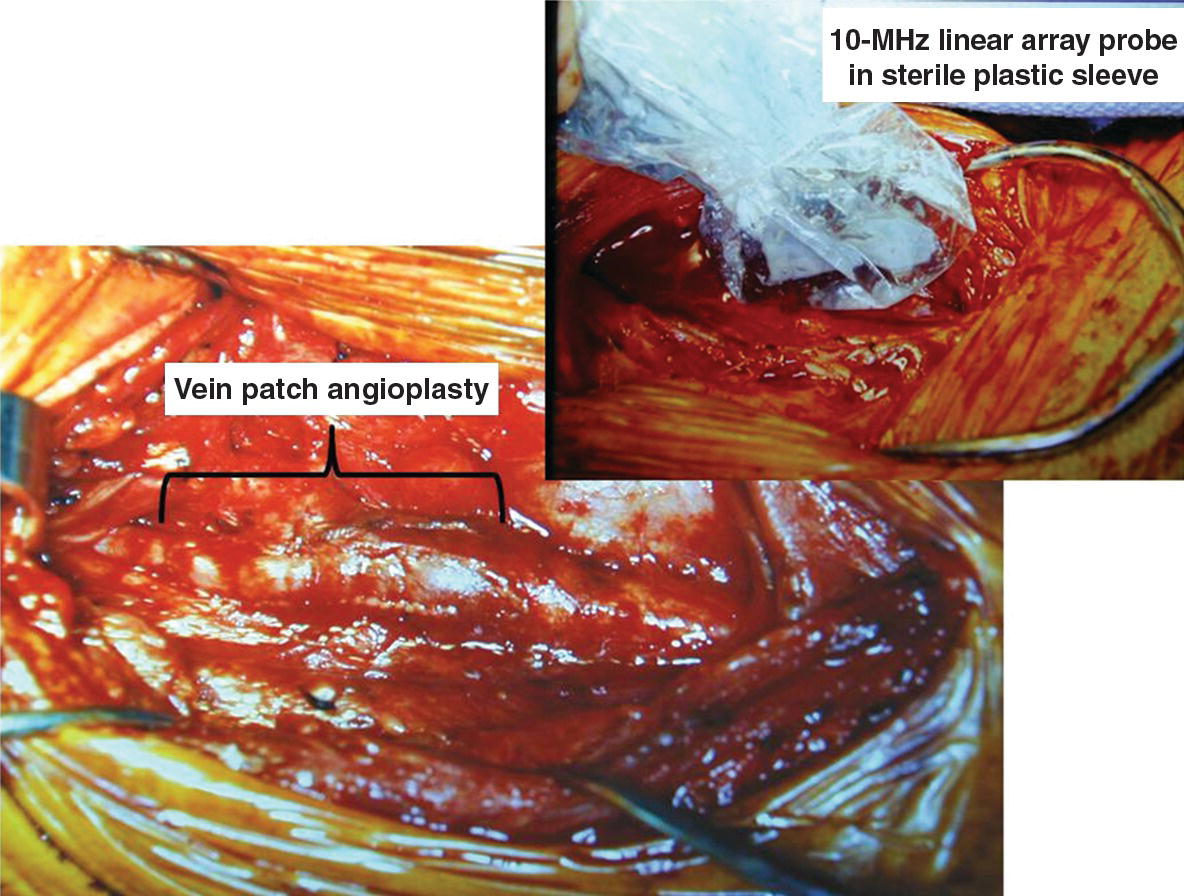
FIGURE 30.2. A vein patch carotid endarterectomy and placement of a small footprint 12-MHz linear array transducer (enclosed in a sterile plastic sleeve) in the neck wound for intraoperative assessment.
Interpretation Criteria
The interpretation algorithm for intraoperative duplex ultrasonography is similar for all types of arterial interventions (Fig. 30.3). Testing is performed after clinical assessment indicates a patent repair and normal pressure pulsation. If B-mode imaging detects lumen abnormalities, velocity spectral recordings are used to document the presence and severity of disturbed flow, that is, systolic velocity increase with spectral broadening. Anatomic defects with no or minimal flow disruption can be observed, but residual stenosis should be corrected. The interpretation criteria for repair site revision are similar for carotid, renal, and peripheral arterial interventions (Table 30.3). In general, an anatomic defect associated with a PSV greater than 150 to 180 cm/s and Vr across the site greater than 2.5 indicates a hemodynamically significant lesion with 50% or greater diameter reduction (DR). Velocity spectra with PSV greater than 300 cm/s signify a pressure-reducing, flow-reducing stenosis, and if lumen thrombus is imaged, platelet thrombus may have developed.1,3
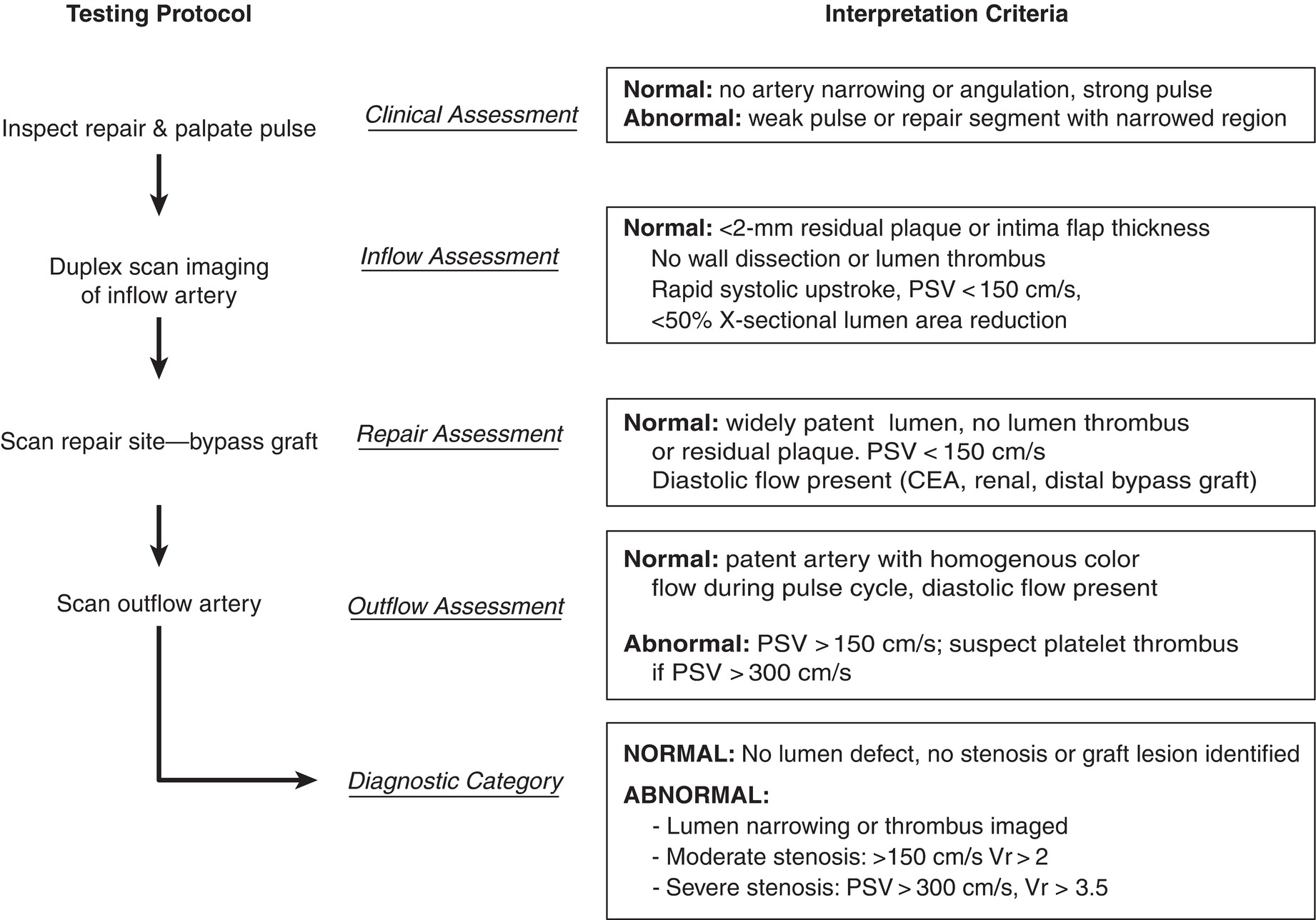
FIGURE 30.3. Interpretation pathway for intraoperative duplex scanning. CEA, carotid endarterectomy; PSV, peak systolic velocity; Vr, velocity ratio.
TABLE 30.3 Interpretation Criteria for Clinically Significant Anatomic LESIONS and Residual Hemodynamic Stenosis Relative to Arterial Repair Site and Type
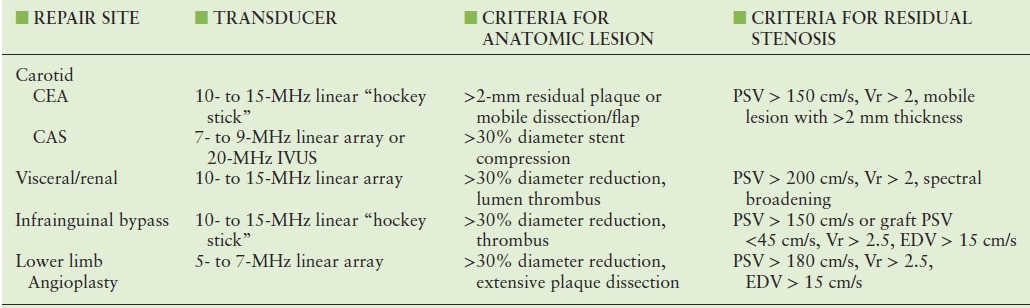
CEA, carotid endarterectomy; CAS, carotid stent angioplasty.
The most common anatomic lesions identified by duplex testing consist of residual atherosclerotic plaque, stenosis, dissection, platelet-thrombus aggregates, intimal-media flaps, retained valve leaflets, and vessel kinks or compression.1–5 When an anatomic defect is detected, Doppler spectral waveforms are recorded across the arterial segment with measurements of PSV and Vr. Spectral waveforms are also inspected for dampening (prolonged acceleration time), changes in phasicity, and spectral broadening that indicates turbulent flow. Flaps more than 2 mm in length, wall dissection, and intraluminal thrombus should prompt repair.2,8 Whenever an abnormality is identified and revised, the arterial segment should be rescanned to confirm a technically satisfactory result.
CAROTID ENDARTERECTOMY/STENT ANGIOPLASTY
Duplex ultrasound is the preferred method to verify that the carotid endarterectomy repair site is technically adequate.2,18 Testing is performed immediately after closure of the arteriotomy and restoration of internal carotid artery (ICA) flow. The carotid repair is imaged beginning in the proximal common carotid artery (CCA) and slowly moving the transducer along the CCA and ICA to the most distal extent possible, including all sites of vascular clamp placement. Use of prosthetic patching, especially polytetrafluoroethylene (PTFE) material, can interfere with lumen imaging due to air in the patch material. Air may persist in the interstices of the prosthetic patch material for days, but it is still possible to scan through nonpatched artery wall, evaluate for lumen defects, and obtain center-stream pulsed Doppler velocity waveforms. The most common anatomic abnormalities identified are CCA plaque and ICA suture line stenosis. Plaques with greater than 2 mm thickness (that is, a “shelf”), focal dissection with flow around residual plaque, and clamp injuries should be repaired. Fronds of the intima that move with each cardiac cycle are typically not associated with a flow disturbance and do not require repair. The majority (>90%) of studies will confirm a “normal” repair (Fig. 30.4) with a widely patent lumen and PSV less than 150 cm/s in the ICA at the endarterectomy end point.1,2 Interpretation criteria for a “significant” stenosis include an anatomic defect with a PSV greater than 150 cm/s and Vr greater than 2 (Fig. 30.5). Exploration of the carotid repair will typically confirm an anatomic defect that can be corrected by additional endarterectomy, patch angioplasty, or patch extension. After revision, repeat duplex testing should be performed to verify that the abnormality has been corrected and no new defects have occurred. The decision to repair an external carotid artery (ECA) stenosis (PSV > 300 cm/s) or occlusion depends on surgeon bias regarding the clinical importance of maintaining patency in this branch. Typically, residual plaque in the ECA can be removed through a transverse incision at the origin of this vessel without clamping the CCA or ICA.
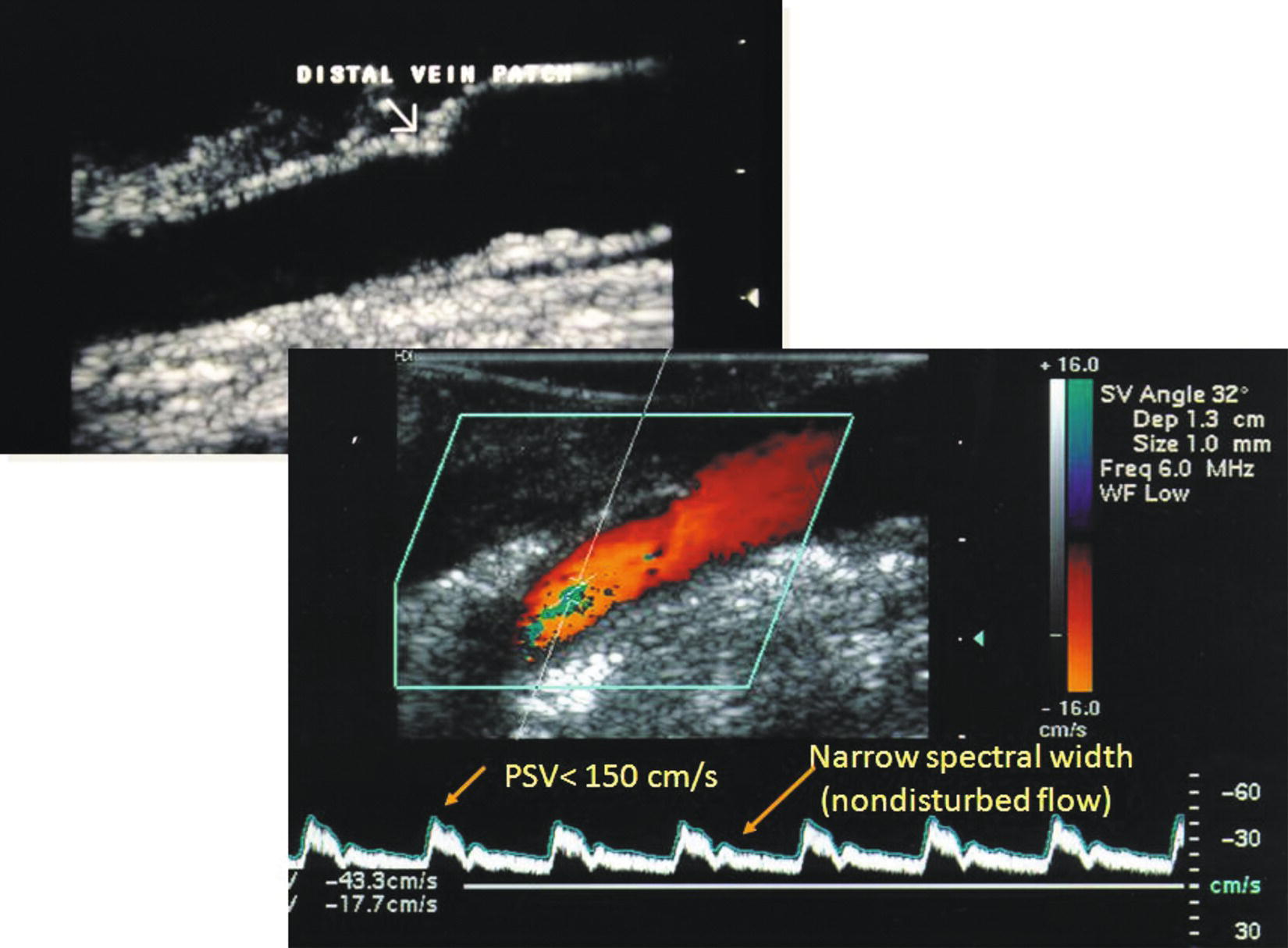
Duplex ultrasound of a vein patch carotid endarterectomy site with a normal B-mode image (top) and pulsed Doppler velocity spectral waveform (bottom) in the distal patch and internal carotid artery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


