The reported incidence of any degree of kidney dysfunction after aortic aneurysm surgery ranges from 0% to 40% depending on the criteria used and the type of aneurysm repaired, and is it irreversible in about 4%. More importantly, the patients who do suffer kidney failure after AAA repair and subsequently require dialysis have about a 70% mortality (regardless if it occurred after a ruptured AAA or not) even if they later recovered kidney function. The baseline patient characteristics with the highest risk factors for postoperative kidney failure after AAA repair are preexisting kidney dysfunction (odds ratio [OR], 2.75) and age greater than 75 years (OR, 1.6). Patients with a baseline creatinine greater than 1.5 g/dL have been identified to be at special risk in all types of aortic surgery. Thus, before aortic surgery, explicit discussions about the risk of kidney failure should be included when discussing informed consent with patients at risk for kidney failure. In addition, even more attention is needed to avoid any additional insults to their kidneys and volume status during their procedure and recovery.
It is intuitive that the placement of a suprarenal aortic cross clamp will lead to some degree of ischemia and reperfusion of the kidney, which could subsequently lead to acute tubular necrosis. Considering this, it is not surprising that the repair of juxtarenal and suprarenal aortic aneurysms have the highest risk of postoperative kidney failure and mortality. The duration of a suprarenal clamp appears to be proportional to the risk of kidney failure and death, particularly if the total clamp time exceeds 100 minutes. In cases where renal artery reimplantation or bypasses are also necessary, the risk of kidney failure is almost three times higher than in patients in whom only an aortic anastomosis is necessary.
Some surgical techniques that can ameliorate renal ischemia when the renal arteries are intimately involved or need revascularization during aortic surgery are worth mentioning. If the aortic neck is deemed to be too short for an infrarenal clamp, then both renal arteries should be dissected to determine if there is enough space to place the clamp distal to at least one of the renal arteries and preserve antegrade blood flow to at least one. If a prolonged suprarenal cross-clamp time is anticipated, then there are two possible interventions to minimize renal ischemia. One is to perform the renal artery revascularization using inflow that is proximal or distal to the AAA using the supraceliac aorta or the iliac arteries, before addressing the complex aneurysm. Another is pre-sewing the renal graft off the intended aortic graft (or using a prefabricated branched graft) before any cross clamp and subsequently performing the distal aortic anastomosis first, followed by the renal artery graft anastomosis. This allows retrograde flow to perfuse the kidneys to minimize the warm ischemia time.
Another technique initially described by Lachat and colleagues is the Viabahn open revascularization technique (VORTEC). VORTEC includes a hybrid approach that combines a branch graft that was sewn onto an aortic graft being connected to the renal artery by way of a short stent graft. After minimal dissection of the renal artery, access is gained into the renal artery with a wire exiting the side of the artery. A stent graft is then deployed antegrade through a sheath placed inside the intended renal artery bypass graft with minimal interruption of flow. The stent graft is then ballooned open and fixed in position with interrupted sutures, and the hole for the sheath is closed. This may be best used in patients whose renal arteries cannot be properly exposed and whose arteries are of sufficient caliber and length to maintain a patent stent graft. Interest in this hybrid technique for anastomosis involving the viscera and lower extremities has been such that there is now a commercially available expanded polytetrafluoroethylene (ePTFE) graft where part of it is a covered stent graft and part of it is a normal ePTFE.
Inadvertent injury to the renal arteries during their exposure, or as a result of an injury created by the occlusion device (vascular clamps, atraumatic clips, or vessel loops) also should be minimized and recognized as early as possible. Intraoperative Doppler evaluation of the renal arteries before clamping, and again after the clamps have been removed (while the patient is still anticoagulated), facilitates early detection of injuries and their repair. Placing undue tension with vessel loops on the renal arteries leads to an increased risk of dissection or plaque disruption. Division of the left renal vein has been studied in large case series and has not been shown to lead to kidney dysfunction. Therefore, provided there is no suspicion of renal venous hypertension, the left renal vein may be divided medial to the adrenal vein and close to the vena cava as indicated.
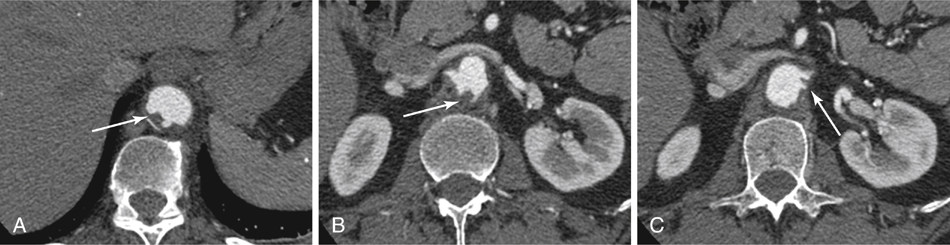
Because postoperative kidney failure can also occur after infrarenal clamping, direct interruption of aortic flow from a clamp is not the only factor involved; the etiology can lie below the origin of the renal arteries. Embolization of soft mural thrombus seen on computed tomography angiogram (CTA) in the perirenal aorta (Figure 1) is likely the most common source for severe kidney failure after an otherwise uncomplicated infrarenal aortic operation. The direct “toothpaste” phenomenon occurs when the aortic clamp squeezes mural atheroma up into the renal arteries. One should identify an area of the aorta for clamping on the preoperative CTA that has minimal thrombus and calcium, even if it is not in the infrarenal aorta. Similarly, one may occlude the renal arteries before placing the aortic clamp in the most normal-appearing aorta (even if it is suprarenal).
In some cases it is reasonable to perform a limited endarterectomy of the loose atheroma in the perirenal aorta, followed by briefly releasing the aortic clamp and allowing the renal arteries to also backbleed into the sac, following which the clamp is placed infrarenally. If there is no suitable suprarenal site that is reasonable to use, then the same flushing technique can be used in a concerning, thrombus-laden infrarenal aorta. Assuming the renal artery is amenable to safe clamping, this technique should prevent embolization into the renal arteries from the cross clamp.
Since the 1980s, there has been much debate over multiple pharmacologic strategies on how to minimize postoperative kidney failure after aortic surgery, especially in aneurysm repair. Most focused on the use of perioperative infusions of antioxidants or diuretic agents for an arbitrary amount of time. These have included furosemide, mannitol, dopamine (and fenoldopam), steroids, N-acetylcysteine, and other antioxidants, all of which have failed to show any significant difference in randomized, controlled trials or meta-analysis. Because many of these strategies lead to increased urine output regardless of their protective effect, postoperative dehydration can paradoxically lead to an increased risk of kidney failure. The only strategy found to be effective in randomized trials to mitigate postoperative kidney failure in cases of suprarenal clamping (mostly studied in thoracoabdominal aneurysms) is directly perfusing cold fluid into the renal artery. Although multiple variations of renal flushes have been promoted by different groups, it seems that the infusion of a simple cold crystalloid solution has yet to be surpassed by any other (including cold blood) when scrutinized in a randomized fashion.
Finally, maintenance of intravascular euvolemia in the postoperative period is critical to avoid kidney failure. The amount of fluids required in the postoperative period should be proportional to the magnitude of the laparotomy. Cases where there was extensive lysis of adhesions, an inflammatory or infected aneurysm, a prolonged period of time where the bowel was compressed by retractors, or any other condition where one would anticipate that there will be a large amount of transudate requires a proportional increase in postoperative fluid resuscitation. Once again, if diuretics (loop or osmotic) were given in the operating room, then care must be taken to appropriately supplement fluids so as to avoid intravascular depletion. Other adjuncts useful to monitor the patient and avoid prerenal azotemia may include central venous pressure monitoring, cardiac output monitoring, or simply astute clinical observations such as discovering inappropriate tachycardia or hypotension in the setting of an epidural spinal catheter or disproportionately high urine output following diuretics.
Recalling that prolonged aortic clamp times alone are associated with renal failure and mortality, a study by Miller in thoracoabdominal aneurysms suggested that even when renal perfusion was maintained to the kidney using extracorporeal pumps, clinically underappreciated rhabdomyolysis can also contribute to postoperative renal failure. A high index of suspicion in patients with marginal lower extremity blood supply should be maintained, and elevated serum myoglobin or creatine kinase can indicate the etiology of otherwise unexplained kidney failure after aortic reconstructions.
Even after avoiding renal ischemia and reperfusion, avoiding atheroembolism, and maintaining euvolemia, the systemic inflammatory response associated with major aortic surgery might ultimately be the cause of postoperative renal failure. This most underappreciated etiology includes the role of cytokines and inflammatory mediators, which can injure the kidney. The most studied in the literature include interleukins 1β, 6, and 8 and tumor necrosis factor α. This is best exemplified by the highest risk of kidney failure and death associated with repairs of inflammatory aortic aneurysm and ruptured aortic aneurysm.
![]()



