19 Interventions in Cardiogenic Shock
 Background
Background
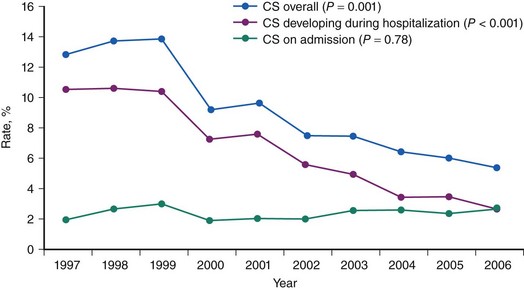
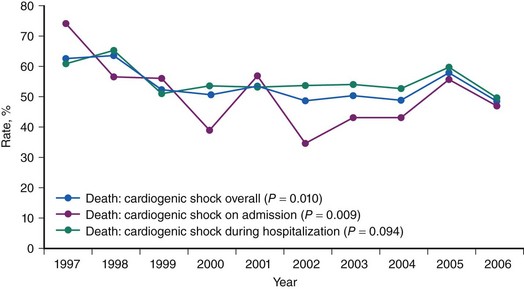
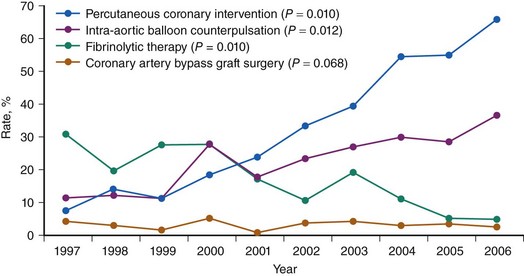
CS is the leading cause of mortality among patients hospitalized for AMI.1 The incidence and mortality of patients with CS in the setting of AMI have remained unchanged for nearly two decades, despite a progressive decline in the overall mortality of AMI, with the development of the coronary care unit and early defibrillation in the 1970s, the routine use of aspirin and β-blockers in the 1980s, and the introduction of reperfusion therapy with thrombolytics in the early 1990s.2–6 More recently, however, registries collecting data from the mid-1990s have shown a decrease in the incidence and mortality of CS complicating AMI (Figs. 19-1 and 19-2).7,8 These trends are associated with the increasing use of primary PCI and rescue PCI for STEMI (Fig. 19-3). To understand which of the patients with shock complicating AMI benefit from PCI, it is necessary to define CS, review its pathophysiology, and examine in detail the trials of PCI in shock.
 Definition and Pathophysiology
Definition and Pathophysiology
CS is a state of end-organ hypoperfusion caused by cardiac failure. Manifestations may include cold extremities, decreased urine output (less than 30 mL/hr), alteration in mental status, or both in the setting of low systemic arterial blood pressure. Hemodynamically, CS is characterized by low cardiac output and systemic arterial hypotension in the setting of normal or elevated left ventricular filling pressure. Typical hemodynamic parameters of CS are shown in Table 19-1.
TABLE 19-1 Typical Hemodynamic Parameters of Cardiogenic Shock
| Cardiac Index | Less Than 2.2 L/M2 |
|---|---|
| Systolic blood pressure | <90 mm Hg with supportive measures, or mean arterial pressure 30 mm Hg less than baseline |
| Left ventricular filling pressure | LVEDP > 15 mm Hg |
L/m2, liters per meters squared; LVEDP, left ventricular end-diastolic pressure.
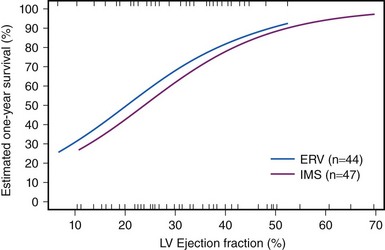
CS results from temporary or permanent derangements in the entire circulatory system. Many of these abnormalities are partially or completely reversible, which may explain the positive functional outcome in most survivors. Left ventricular pump failure is the primary insult in most forms of CS. Left ventricular dysfunction may reflect new irreversible injury, reversible ischemia, damage from prior infarction, or a combination of these. The decrease in coronary perfusion as a consequence of the low cardiac output may compromise flow in vessels other than the infarct artery, which leads to ischemia in the territory of the non–infarct-related artery and further decline in left ventricular function. The degree of myocardial dysfunction that initiates CS is variable. It is often, but not always, severe. Thus, other components of the cardiovascular system contribute to the pathophysiology of CS in the setting of AMI. The decrease in cardiac output triggers the release of catecholamines, which constrict the peripheral arterioles to maintain the perfusion of vital organs. This reflex mechanism of increased systemic vascular resistance is not fully effective, as demonstrated by variations in the systemic vascular resistance in trials of CS, in which hemodynamic data were assessed, with median values during CS in the normal range despite vasopressor therapy.9 These findings are consistent with the observation that MI can cause systemic inflammatory response syndrome (SIRS). Right ventricular dysfunction may also cause or contribute to CS. Predominant right ventricular failure is rare and represents only 5% of cases of shock complicating AMI. Right ventricular failure may limit left ventricular filling via a decrease in cardiac output, ventricular interdependence, or both. Shock caused by isolated right ventricular dysfunction carries nearly as high a mortality risk as shock caused by left ventricular failure. Additionally, the benefit of revascularization was similar in the SHOCK trial and registry in patients with primarily right ventricular dysfunction versus primarily left ventricular dysfunction.10 Mechanical complications such as ventricular septal rupture and papillary muscle rupture may lead to CS without severe reduction in left ventricular function.11,12 Finally, most patients with CS complicating AMI develop shock after presenting at the hospital. In some, medication use contributes to the development of shock. Classes of medications used to treat AMI that have been associated with shock include β-blockers, angiotensin-converting enzyme (ACE) inhibitors, nitrates, diuretics, and morphine. In light of the complex pathophysiology of CS, severe impairment of left ventricular contractility does not always lead to shock, and conversely, left ventricular ejection fraction (LVEF) may be only moderately depressed in patients with CS. In fact, the mean LVEF in the SHOCK trial was 30%.13 However, LVEF remains an important prognostic indicator (Fig. 19-4).14
 Clinical Presentation
Clinical Presentation
SHOCK Trial and Registry
The SHOCK trial is the largest randomized trial comparing immediate revascularization (accomplished by either PCI or coronary artery bypass grafting [CABG]) and initial medical stabilization (including reperfusion therapy with thrombolytics) in the setting of CS complicating AMI.13,15 This trial and its registry provide insight into the etiology, risk factors, hemodynamic profile, clinical features, timing, and prognosis of CS complicating AMI. Eligible patients had to have an ST segment elevation myocardial infarction (STEMI) complicated by shock caused by left ventricular failure (mechanical and iatrogenic causes excluded), clinical and hemodynamic confirmation of CS, and shock developing within 36 hours of infarction. Intra-aortic balloon counterpulsation was permitted in both arms. Randomization had to occur within 12 hours of the diagnosis of shock. Patients with severe systemic illness, mechanical complications of MI, dilated cardiomyopathy (DCM), and severe valvular heart disease were excluded. A total of 302 patients were randomized in the SHOCK trial. A simultaneous registry prospectively enrolled patients who did not fulfill the eligibility criteria for entry into the trial. The SHOCK trial and registry included patients with mechanical complications of AMI, patients with shock secondary to medications, patients who presented more than 36 hours after infarction or were not randomized within 12 hours of shock, and patients with shock diagnosed on clinical grounds alone (no hemodynamic confirmation). A total of 1190 patients were enrolled in this prospective, nonrandomized registry.