17 Intervention for Non-ST-Segment Elevation Acute Coronary Syndromes
 Introduction
Introduction
Over the past 31 years percutaneous coronary intervention (PCI) has evolved from a somewhat tenuous and experimental procedure to a durable and mainstream therapy; as a result, the clinical indications for PCI have broadened to include patients with both stable angina pectoris and acute coronary syndrome (ACS).1–3 ACS comprises the spectrum of clinical signs and symptoms that occur as a result of acute myocardial ischemia and is classified as a non-ST-segment elevation ACS (NSTE-ACS) or as an ST-segment elevation ACS (STE-ACS). NSTE-ACS includes patients with unstable angina (UA) or non-ST-segment elevation myocardial infarction (NSTEMI); STE-ACS includes patients with ST-segment elevation myocardial infarction (STEMI)4 Various strategies have been proposed for the management of NSTE-ACS, with more recent guidelines and clinical trials increasingly supporting an invasive strategy for high-risk ACS and a less invasive/more conservative strategy for patients deemed to be at lower risk.5 This chapter discusses PCI for patients with NSTE-ACS, focusing on the pathophysiology, risk stratification, adjunctive treatment during PCI, invasive versus conservative strategy, and the most recent AHA/ACC guidelines for the management of patients with NSTE-ACS.
 Background and Rationale for Percutaneous Coronary Intervention in Patients with Non-St-Segment Elevation Acute Coronary Syndrome
Background and Rationale for Percutaneous Coronary Intervention in Patients with Non-St-Segment Elevation Acute Coronary Syndrome
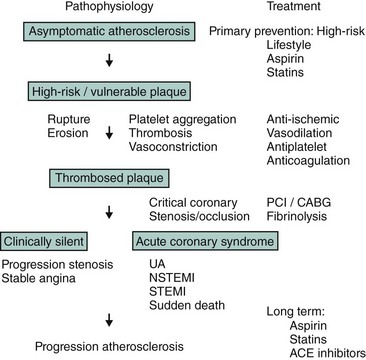
Coronary atherosclerosis is a chronic disease in which atheromatous material generally evolves silently over time; it may eventually result in the development of a high-risk (i.e., vulnerable) plaque6 (Fig. 17-1). Rupture or erosion of this high-risk plaque triggers the formation of intracoronary thrombosis, which can lead to a critical stenosis or occlusion of the coronary artery as well as associated vasospasm. Although the initial stenosis may evolve silently, healing of a ruptured or eroded plaque may lead to more rapid progression of the stenosis; this may remain clinically silent or cause angina pectoris. Thus ACS may have different clinical presentations: UA, NSTEMI, STEMI, and sudden death. All clinical manifestations of ACS share a common pathophysiological pathway (plaque rupture/erosion and some degree of thrombosis and vasoconstriction), but the duration (transient or permanent) and severity (subtotal or total coronary occlusion) are different and associated either with myocardial necrosis (STEMI and NSTEMI) or no evidence of myocardial necrosis (UA) as manifest by negative cardiac biomarkers. It is important to note that within the first few months after an initial episode of coronary instability, there is a strong tendency for repeat instability caused by progression in the severity of the culprit stenosis or that of remote lesions. Although plaque rupture or erosion with associated thrombosis is the most common cause of ACS, it may also be caused by dynamic obstruction (i.e., Prinzmetal’s angina or vasospasm secondary to drug abuse), spontaneous coronary artery dissection (most commonly occurring in peripartum women), severe coronary narrowing without thrombus or spasm (i.e., advanced progressive atherosclerosis or severe restenosis from prior PCI), or a precipitating condition extrinsic to the coronary circulation (i.e., fever, sepsis, tachycardia, hypotension, anemia, hypoxemia, etc.).5 Thus in evaluating individual patients with ACS, it is important to consider the most likely etiology, especially if an invasive strategy is being considered, given that PCI is not necessarily an appropriate therapy for all causes of ACS. Patients with NSTE-ACS have long-term outcomes similar to those of patients with STE-ACS and worse than those of patients with unstable angina.7 NSTE-ACS patients and STE-ACS patients have similar prognoses, likely due to a high prevalence of multivessel disease as well as a higher incidence of recurrent ischemia (35% vs. 23% at 1 year in the GUSTO-IIb trial). The optimal management of unstable angina or NSTEMI, therefore, is twofold: immediate relief of ischemia and the prevention of progression to acute MI or cardiac death. This can be achieved by a combination of anti-ischemic, antiplatelet, and antithrombotic therapy with or without PCI (Fig. 17-1).
Risk Stratification
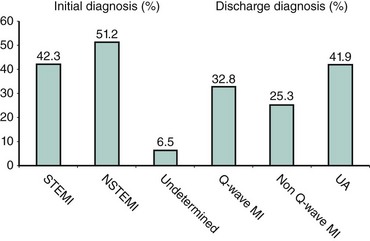
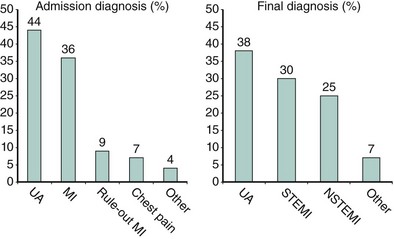
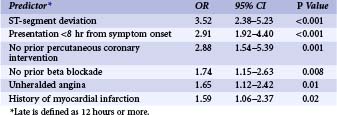
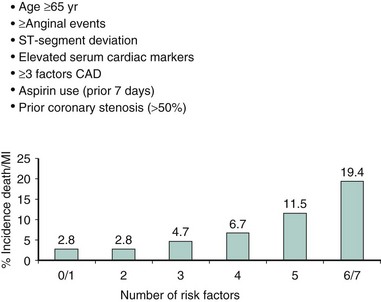
Although the risks of individual patients presenting with NSTE-ACS vary widely, several risk scores have been devised to risk-stratify patients and help identify those who may benefit from an early invasive strategy. The TIMI (Thrombolysis in Myocardial Infarction) risk score, which predicts the risk of 14-day all-cause mortality and new or recurrent MI, is a validated risk-prediction model based on data from the TIMI IIB and ESSENCE trials and is the most commonly used. Seven variables at presentation were independently predictive of outcome (Fig. 17-2).8 The GRACE Registry developed a risk calculator for bedside risk estimation of 6-month mortality for patients after hospitalization for ACS.9 The overall 6-month mortality rate was 4.8%. Nine predictive variables were identified: older age, previous MI, history of heart failure, increased heart rate, lower systolic blood pressure, serum creatinine level, elevated levels of cardiac biomarkers, ST-segment depression, and no PCI (Figs. 17-3A and 17-3B). The presence of elevated cardiac biomarkers is a particularly important prognostic indicator, which has been associated with an increased risk for reinfarction and death (discussed further below). It is important to note that although determination of patient risk is vitally important upon initial presentation with NSTE-ACS, individual patient risk often changes during hospitalization and it should be frequently reassessed.
 Predicting a Late Positive Serum Troponin Level in Initially Troponin-Negative Patients with Non-ST-Segment Elevation Acute Coronary Syndrome
Predicting a Late Positive Serum Troponin Level in Initially Troponin-Negative Patients with Non-ST-Segment Elevation Acute Coronary Syndrome
A limitation of the use of the TIMI risk score in identifying patients at higher risk who present with NSTE-ACS is that some patients may initially present with negative cardiac biomarkers, only to demonstrate elevation in these markers 12 hours later. Of 1,342 patients who were enrolled in the TIMI-IIIB trial, 200 (14.9%) were troponin-negative at baseline but developed an elevated troponin I level (≥0.4 ng/mL) at 12 hours.10 Six independent predictors were identified (Table 17-1), and a score was derived to identify patients with the highest likelihood to become troponin-positive later during hospital admission (Fig. 17-4). The derived score was tested in 855 patients in the GUSTO IIA (Global Use of Strategies To Open Occluded Arteries in Acute Coronary Syndromes IIA) study and similarly predicted a late rise in troponin T levels. This score, therefore, may be useful to further assist in the risk-stratification of patients who present with NSTE-ACS and initially unremarkable troponin levels.
 Adjunctive Treatment during Percutaneous Coronary Intervention for Non-ST-Segment Elevation Acute Coronary Syndrome
Adjunctive Treatment during Percutaneous Coronary Intervention for Non-ST-Segment Elevation Acute Coronary Syndrome
Antiplatelet Treatment
The activation and aggregation of platelets after the rupture of a vulnerable plaque are key components in the pathophysiology of ACS. Aspirin inhibits the cyclooxygenase pathway in platelets and in the endothelium, which prevents the production of thromboxane A2 and therefore inhibits platelet aggregation.11,12 Multiple studies have shown the benefit of aspirin therapy in coronary artery disease with reduction in angina, death, and MI by 30%.13,14 Two metanalyses have established that aspirin has efficacy across a wide range of dosages.13,14 Currently, it is recommended that a dosage of between 162 and 325 mg be given orally on a daily basis beginning at the acute presentation and continuing for 1 month after PCI when a bare metal stent is used and 3 to 6 months when a drug-eluting stent is used.5 Thereafter, the dosage may be reduced to between 81 and 150 mg daily on an indefinite basis unless a significant contraindication exists. Although aspirin is certainly an important pharmacological therapy in ACS patients, it does not prevent all thrombotic events.
Thienopyridines—such as ticlopidine, clopidogrel, and prasugrel—exert their antiplatelet effects by blocking the P2Y12 receptor and its associated signaling pathway, thereby inhibiting platelet activation. Thienopyridines are administered as an initial loading dose, followed by a maintenance dose because they take longer to exhibit their irreversible antiplatelet effect. It is important to note that their mechanism of action is entirely independent of and complementary to that of aspirin, and the combination of aspirin and a thienopyridine is superior to the use of aspirin alone.15,16 Ticlopidine in conjunction with aspirin was shown to reduce the rate of vascular death and MI by 46% in NSTE-ACS.17 Clopidogrel is the most widely studied of the thienopyridine class. The CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events) trial18 studied 19,185 patients with atherosclerotic vascular disease and revealed a 9% relative risk reduction in vascular death, MI, or ischemic stroke without a significant increase in bleeding. The CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) trial15 enrolled 12,562 patients and demonstrated a 20% reduction in cardiovascular death, MI, and stroke. A subset of this population who underwent PCI were studied in the PCI-CURE trial,19 which showed that pretreatment with clopidogrel for a median of 6 days before PCI was associated with a 31% reduction in cardiovascular death or MI. The optimal dosing regimen for clopidogrel is still somewhat a matter of debate. A loading dose of 600 mg achieves maximal platelet inhibition faster than 150 or 300 mg and has lasting benefit of up to 30 days in low- to intermediate-risk patients undergoing elective PCI20; however, in higher-risk patients (discussed in detail below), the use of glycoprotein IIb/IIIa inhibitors has an added benefit. An important consideration for the use of clopidogrel in ACS patients is the substantial variability in individual patient response to this drug. Multiple potential hypotheses have been proposed to explain this “clopidogrel resistance,” such as differences in clopidogrel dosing, intestinal absorption problems, and varying availability and clearance of the active metabolite.21 Genetic factors such as polymorphisms of the hepatic CYP pathway (which is responsible for metabolizing clopidogrel’s prodrug into an active metabolite) are becoming increasingly well understood and used in mainstream clinical practice.22 Testing for CYP mutations, especially of CYP3A, as well as platelet function testing are increasingly being utilized to direct individual patient care.23 Prasugrel is a newer-generation P2Y12 inhibitor that is more potent and more consistent with respect to platelet inhibition. The TRITON-TIMI 38 (Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38) trial24 evaluated 13,608 patients with moderate to high risk ACS (NSTE-ACS and STE-ACS) after randomly receiving either prasugrel or clopidogrel during PCI. In this study, prasugrel significantly reduced the composite endpoint of cardiovascular death, nonfatal MI, or nonfatal stroke by 19% as compared with clopidogrel. The TRITON trial showed that prasugrel was associated with a 24% reduction in MI, a 34% reduction in the need for urgent revascularization, and a 52% reduction in stent thrombosis.25 These benefits, however, were also associated with a 0.5% absolute increase in non-coronary artery bypass graft surgery (non-CABG)-related TIMI major bleeding, a fivefold increase in CABG-related bleeding, and a 0.3% absolute increase in fatal bleeding24 (Fig. 17-5). A landmark analysis of this trial revealed that patients with a previous transient ischemic attack (TIA) or stroke, those ≥75 years of age, and those who weighed <60 kg were at especially high risk of bleeding.25–26 Dual antiplatelet therapy with aspirin and a thienopyridine is considered standard pretreatment for patients with NSTE-ACS undergoing PCI with or without stent implantation (ACC/AHA guidelines class 1 recommendation, level of evidence A)5; however, the optimal timing for initiation of thienopyridine therapy may still pose a dilemma in actual clinical practice. The frequency of adverse cardiac events is reduced within the first hours of treatment with a thienopyridine; but if the patient is referred for urgent or emergent surgery, this approach may be associated with more perioperative blood loss.27 However, given the fact that CABG is less likely to be necessary in the contemporary stent era even for high-risk NSTE-ACS patients, early thienopyridine treatment is recommended unless urgent CABG is deemed to be very likely.
Anticoagulant Treatment
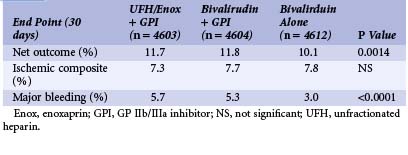
Since the beginning of PCI, unfractionated heparin (UFH) has been given to prevent thrombosis during intracoronary instrumentation and to minimize thrombosis at the site of the plaque, which is damaged by balloon angioplasty and/or stent implantation. Treatment with UFH in addition to aspirin is usually given based on the data from a metanalysis demonstrating a reduction of the combined death and MI rate of 7.9% for those treated with UFH compared with 10.3% for those treated with aspirin alone.28 UFH is given as an initial intravenous bolus, usually as 100 IU/kg, and guided by the activating clotting time (ACT) in the range of 250 to 300 seconds. If combined with a GP IIb/IIIa inhibitor, the dose of UFH should be lower: initial intravenous bolus of 50 to 60 IU/kg and ACT in the range of 200 to 250 seconds to prevent excessive bleeding complications. Although the ACC/AHA guidelines recommend that patients with NSTE-ACS receive heparin, the actual duration of heparin therapy is not well established, and most trials that have examined UFH recommend 2 to 5 days of therapy.5 Low-molecular-weight heparin (LMWH) has also been used in the setting of PCI for NSTE-ACS. In the FRISC (Fast Revascularization during Instability in Coronary Artery Disease) trial,29 1,506 patients were randomly assigned to receive dalteparin (120 IU/kg twice daily, with a maximal dose of 10,000 IU) or UFH during the first 5 to 7 days of hospitalization followed by dalteparin (7,500 IU subcutaneously, daily) or placebo for 35 to 45 days. Dalteparin was associated with a 63% relative risk reduction in death or MI during the first 6 days (1.8% in the treatment group versus 4.8% in the placebo group), with persistence of these differences at 40 days. The ESSENCE (Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-wave Coronary Events) trial30 evaluated 3,171 UA/NSTE-ACS patients who were randomly assigned to receive enoxaparin (1 mg/kg twice daily) or continuous infusion of UFH (minimum of 48 hours to a maximum of 8 days). The risk of recurrent angina, MI, or death was significantly lower in the enoxaparin patients than in the UFH patients at 14 days (16.6% vs. 19.8%). The benefit persisted at 30 days; however, this came at the cost of increased minor bleeding but no significant difference in major bleeding (6.5% vs. 7%).30 The TIMI IIB (Thrombolysis In Myocardial Infarction) trial31 randomized 3,910 patients with UA/NSTE-ACS to receive either enoxaparin or UFH for 3 to 8 days while hospitalized, followed by either enoxaparin or placebo through day 43 on an outpatient basis. At 8 days, a 14.6% risk reduction in the composite endpoint of MI, death, and need for urgent revascularization was noted, and at 43 days a 12.3% risk reduction in the same endpoint was reported. Bleeding was similar in both groups during initial hospitalization, but the risk of major bleeding during the outpatient phase of the study was doubled in the enoxaparin group as compared with placebo. These data suggest that enoxaparin may be more effective than UFH at reducing the risk of ischemic events during the acute management of NSTE-ACS patients without an important increase in major bleeding events. A metanalysis of nearly 22,000 patients with UA/NSTE-ACS enrolled in six randomized trials reported a relative risk reduction of 9% in the composite endpoint of death or MI at 30 days for patients treated with enoxaparin as compared with those treated with UFH and no significant difference in major bleeding at 7 days.32 These results demonstrate that the use of enoxaparin was beneficial when an early conservative strategy was utilized for patient management. More recent trials have compared UFH with enoxaparin when an early invasive strategy is implemented. The SYNERGY (Superior Yield of the New Strategy of Enoxaparin, Revascularization and Glycoprotein IIb/IIIa Inhibitors) trial33 studied 10,027 patients with high-risk UA/NSTE-ACS who were treated using an early invasive strategy. In this study, there was no significant difference in the primary endpoint of all-cause mortality or nonfatal MI at 30 days, but patients who received enoxaparin exhibited a 20% increase in TIMI major bleeding in association with invasive procedures, especially CABG.33 When considered as a whole, the data presented above support the notion that treatment with enoxaparin as compared with UFH appears to be more efficacious at reducing ischemic events in UA/NSTE-ACS patients who are treated with an early conservative strategy. However, it may also result in increased bleeding with invasive procedures. Interpretation of data from the SYNERGY trial and other trials comparing UFH and enoxaparin in UA/NSTE-ACS patients treated with an early invasive strategy must be interpreted carefully, given that many of the patients in these trials had already received one or more other antithrombotic agents before being randomized to a given treatment arm. Thus the high rate of patient crossover is an important confounding factor. The most recent ACC/AHA guidelines consider enoxaparin to be a reliable alternative to UFH for patients treated both with an early conservative and an early invasive strategy (class 1, level of evidence A).5 Bivalirudin is a direct thrombin inhibitor and a synthetic analog of hirudin. It reversibly binds thrombin and inhibits clot-bound thrombin. The REPLACE II (Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events II) trial34 demonstrated that bivalirudin could be used as an alternative to UFH plus GP IIb/IIIa blockade in patients at low risk who undergo PCI. The efficacy of bivalirudin was further evaluated in the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial.35 It randomized 13,800 patients with moderate- to high-risk unstable angina or NSTEMI undergoing an invasive strategy. There were three treatment groups: group I received UFH or enoxaparin plus GP IIb/IIIa, group II received bivalirudin plus a GP IIb/IIIa inhibitor, and group III received bivalirudin alone. The primary endpoint at 30 days was a net clinical outcome based on an ischemic composite (all-cause mortality, MI, or unplanned revascularization for ischemia) or major bleeding. The net outcome was lower with the bivalirudin-alone strategy, which could be ascribed to a significant reduction in major bleeding in the bivalirudin group (Table 17-2). Fondaparinux is a synthetic polysaccharide that leads to indirect inhibition of factor Xa. Fondaparinux was evaluated in the OASIS-5 (Organization to Assess Strategies for Ischemic Syndromes) trial.36 In this study, 20,078 patients were randomized to treatment with either fondaparinux (2.5 mg/day) or enoxaparin (1mg/kg twice daily) for a mean of 6 days. Approximately 40% of patients underwent PCI and 15% underwent CABG in both groups. Fondaparinux was equivalent to enoxaparin in terms of the primary efficacy endpoint at 9 days (composite of death, MI, or refractory ischemia), and major bleeding at 9 days was significantly lower with fondaparinux than with enoxaparin (2.2% vs. 4.1%; P < 0.001). However, fondaparinux was associated with an increased rate of guide-catheter thromboses; thus it has been recommended that additional anticoagulant with anti-IIa activity also be used.5
Glycoprotein Iib/Iiia Inhibitors
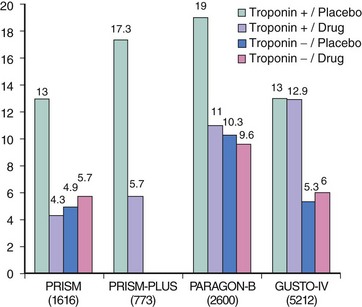
A critical pathway of platelet activation and aggregation is mediated by a conformational change in the glycoprotein IIb/IIIa (GP IIb/IIIa) receptor from an inactive to an active state. GP IIb/IIIa inhibitors interfere with the ability of the GP IIb/IIIa receptor to bind with target ligands; thus they are potent inhibitors of platelet aggregation.37 All three currently used GPIIb/IIIa inhibitors (abciximab, eptifibatide, and tirofiban) exhibit different pharmacodynamic and pharmacokinetic properties, different clinical trial outcomes, and as a result have different recommendations for clinical use. Abciximab was the first of the three currently used GPIIb/IIIa inhibitors to be subjected to large-scale clinical trial testing and was first evaluated in the pre-stent era of the early 1990s. The EPIC (Evaluation of c7E3 [later named abciximab] for the Prevention of Ischemic Complications) trial38 studied high-risk patients with UA, evolving MI, or complex coronary lesion anatomy and showed a 35% reduction in the composite endpoint of death, MI, or recurrent ischemia as compared with placebo. The CAPTURE (c7E3 Fab Antiplatelet Therapy in Unstable Refractory Angina) trial39 studied 1,265 patients with UA who underwent PCI. In this study, a 30% relative reduction in the primary endpoint of all-cause mortality, MI, or recurrent ischemia requiring urgent revascularization was reported at 30 days. The rate of MI was noted to be lower before, during, and after PCI in patients treated with abciximab, and a subgroup analysis revealed that abciximab facilitated thrombus resolution and prevented recurrent ischemia by continuous ECG monitoring. Abciximab therefore has clearly been shown to be efficacious in the setting of UA/NSTE-ACS patients undergoing PCI. The GUSTO-IV-ACS (Global Use of Strategies to Open Occluded Coronary Arteries IV-Acute Coronary Syndrome) trial,40 however, studied 7,800 patients with UA/NSTE-ACS who were not scheduled to undergo an early invasive strategy. Patients in this study were randomized to receive an abciximab bolus followed by infusion for either 24 or 48 hours or placebo. In this trial abciximab provided no benefit with respect to the primary composite endpoint of death or MI at 30 days, even in a subgroup of patients with elevated troponin levels. The current ACC/AHA guidelines reflect these results in that abciximab is not recommended for the treatment of patients with UA/NSTE-ACS for whom an initial conservative strategy is planned.5 Tirofiban was studied in the PRISM (Platelet Receptor Inhibition in Ischemic Syndrome Management) trial,41 in which 3,232 patients with UA were randomized to receive either UFH or tirofiban for 48 hours. A 32% reduction in the rate of death, MI, or refractory ischemia was noted at 48 hours, but no significant difference was noted at 30 days. The PRISM-PLUS (Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms) trial42 randomized UA and NSTE-ACS patients to receive aspirin plus either heparin, tirofiban, or both. The tirofiban-only arm was stopped early owing to excess death at 7 days (4.6% vs. 1.1% in the heparin-only arm). Patients who received both heparin and tirofiban exhibited the greatest benefit in terms of a reduction in the composite endpoint at 7 days of death, MI, or refractory ischemia. This benefit was sustained at 30 days and at 6 months (27.2% vs. 32% in the heparin-only arm). Eptifibatide was studied in 9,461 patients with NSTE-ACS in the PURSUIT (Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrellin Therapy) trial.43 In this study, treatment with eptifibatide was associated with a 10% reduction in the relative risk of death and MI at 30 days. A metanalysis44 confirmed the utility of GPIIb/IIIa inhibitors in the management of patients with moderate- to high-risk UA/NSTE-ACS. This analysis pooled 31,402 patients from six different GPIIb/IIIa trials involving ACS patients and showed a 9% reduction in the odds ratio of death or MI for patients treated with a GPIIb/IIIa inhibitor as compared with placebo. Subgroup analysis revealed a 15% reduction in the odds ratio of death or MI for troponin-positive patients and no such reduction in troponin negative patients. Reduction in the odds ratio of death or MI was also noted to be greater in patients who underwent PCI within 5 days.44 The TARGET (Do Tirofiban and Reopro Give Similar Efficacy Outcomes Trial)45 study was a direct comparison of the efficacy of abciximab with that of tirofiban. It demonstrated that the primary endpoint (composite of death, nonfatal MI, or urgent revascularization at 30 days) was significantly higher in the tirofiban group compared with those receiving abciximab (7.6% vs. 6.0%), but at 6 months, this difference was no longer statistically significant.45 Most recently, the EARLY ACS (Early Versus Delayed Provisional Eptifibatide in Acute Coronary Syndromes) trial46
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree