Interstitial Pattern, Mediastinal-Hilar Adenopathy
Christopher M. Walker, MD
DIFFERENTIAL DIAGNOSIS
Common
Sarcoidosis
Lymphangitic Carcinomatosis
Cystic Fibrosis (Mimic)
Less Common
Silicosis/Coal Worker’s Pneumoconiosis
Usual Interstitial Pneumonia
Rare but Important
Berylliosis
Lymphocytic Interstitial Pneumonia
Diffuse Pulmonary Lymphangiomatosis
Pulmonary Langerhans Cell Histiocytosis
Lymphangioleiomyomatosis
ESSENTIAL INFORMATION
Key Differential Diagnosis Issues
Age of patient
Gender
Race
Presenting symptoms
Helpful Clues for Common Diagnoses
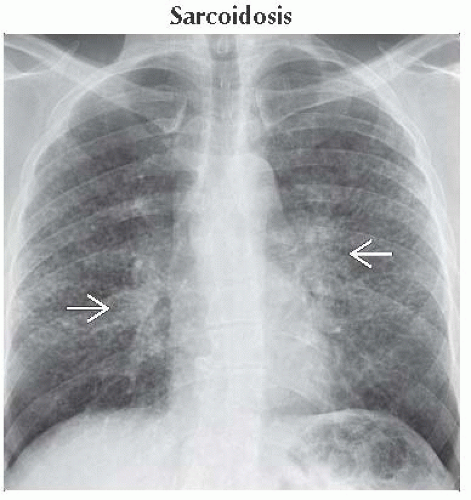
Sarcoidosis
Systemic granulomatous disease of unknown etiology
Common demographics
Women
Child-bearing age
African-American race
Radiography
Symmetric hilar and mediastinal lymphadenopathy
Mid to upper lobe reticulonodular opacities in ≤ 50% of patients
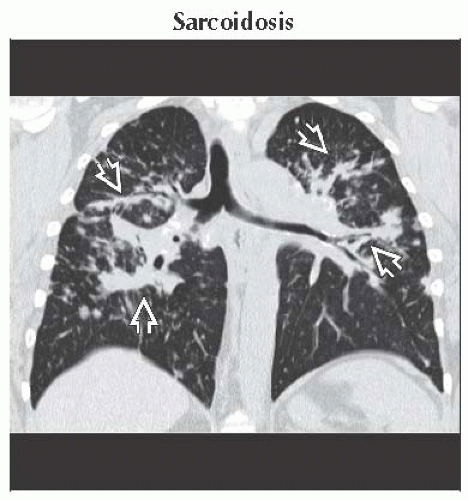
HRCT
Bilateral hilar and mediastinal lymphadenopathy
Perilymphatic nodules involving fissures, subpleural lung, and bronchovascular bundles
Air-trapping secondary to granulomas obstructing small airways
Lymphangitic Carcinomatosis
Tumor growth in pulmonary lymphatics
Common causes include metastases from
Breast carcinoma
Bronchogenic carcinoma
Pancreatic carcinoma
Gastric carcinoma
Thyroid carcinoma
Adenocarcinoma of unknown primary
Unilateral disease occurs most commonly with lung carcinoma
Radiography
Reticulonodular opacities (unilateral or bilateral)
Hilar &/or mediastinal lymphadenopathy
Pleural effusions
HRCT
Nodular or beaded interlobular septal thickening
Perilymphatic nodules
Hilar or mediastinal lymphadenopathy in 30-50% of patients
Pleural effusions
Cystic Fibrosis (Mimic)
Autosomal recessive condition occurring primarily in Caucasians
Results in defective chloride transport across epithelial membranes
Causes variety of problems involving respiratory and gastrointestinal systems
Radiograph
Large lungs with bronchiectasis or bronchial wall thickening mimicking interstitial pattern
Small peribronchovascular nodular opacities secondary to impaction of small airways
Prominent hila indicate
Lymphadenopathy from recurrent infections
Enlarged pulmonary arteries from pulmonary hypertension
Early disease predominates in upper lobes
Uncontrollable hemoptysis may necessitate bronchial artery embolization
Helpful Clues for Less Common Diagnoses
Silicosis/Coal Worker’s Pneumoconiosis
Radiography
Well-circumscribed small nodules predominating in upper lungs
Small percentage of pulmonary nodules may calcify
Nodules may coalesce to form masses with upward retraction of hila; so-called “progressive massive fibrosis”
Hilar lymphadenopathy
HRCT
Centrilobular and subpleural nodules
Posterior upper lung predominance
40% of patients have hilar or mediastinal lymphadenopathy
5% of lymph nodes show peripheral “egg shell” calcification
Usual Interstitial Pneumonia
Basilar and subpleural predominant fibrosis with honeycombing
Mild mediastinal lymphadenopathy in majority of cases
Helpful Clues for Rare Diagnoses
Berylliosis
Identical appearance to sarcoidosis
Perilymphatic nodules, lymphadenopathy, and noncaseating granulomas
Upper lung predominant disease
Key discriminator is occupational exposure to beryllium
Workers in nuclear power, ceramics, aerospace, and electronics
Occurs most commonly 10-15 years after exposure
Most common symptom is dyspnea
Positive beryllium lymphocyte proliferation test via blood or bronchoalveolar lavage
Lymphocytic Interstitial Pneumonia
Strong association with Sjögren syndrome
AIDS defining in children
Diffuse distribution
Poorly defined centrilobular nodules
Diffuse or patchy ground-glass opacity
Isolated or diffuse cystic lung disease
± mediastinal or hilar lymphadenopathy
Diffuse Pulmonary Lymphangiomatosis
Congenital lymphatic disorder
Diffuse smooth interlobular septal thickening
Mild mediastinal lymphadenopathy
50% have associated pleural effusions
Pulmonary Langerhans Cell Histiocytosis
Young male smokers
Radiography
Normal or large lungs
Nodular opacities in upper 2/3 of lung
Interstitial pattern results from “moire effect” of superimposed cysts
Pneumothoraces in 30% of cases
HRCT
Bizarrely shaped cysts
Relative sparing of lung bases
Paracicatricial emphysema
Centrilobular nodules ± cavitation
Lymphangioleiomyomatosis
Women of child-bearing age
Radiography and HRCT
Pleural effusions
Pneumothoraces
Uniformly distributed similar-sized cysts
Interstitial pattern results from “moire effect” of superimposed cysts
Mediastinal or retroperitoneal lymphadenopathy
Image Gallery
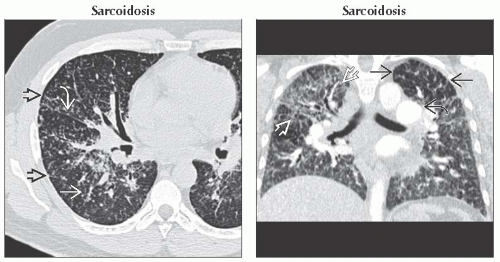 (Left) Axial HRCT shows a perilymphatic distribution of nodules along interlobular septa
 , the major fissure , the major fissure 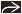 , and bronchovascular bundles , and bronchovascular bundles  . (Right) Coronal CECT shows diffuse interstitial thickening most marked in the right upper lobe . (Right) Coronal CECT shows diffuse interstitial thickening most marked in the right upper lobe 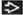 . Enlarged pulmonary arteries are secondary to pulmonary hypertension . Enlarged pulmonary arteries are secondary to pulmonary hypertension  . Lucency in the left upper lobe is secondary to mosaic perfusion from small airways involvement by sarcoid granulomas . Lucency in the left upper lobe is secondary to mosaic perfusion from small airways involvement by sarcoid granulomas  . .Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|