Chronic total occlusion (CTO) in a non-infarct-related artery and chronic kidney failure (CKD) are associated with worse outcomes after primary percutaneous coronary intervention (PCI). The aim of this study was to investigate the interaction of CTO and CKD in patients who underwent primary PCI for acute ST-segment elevation myocardial infarction (STEMI). Patients with STEMIs with or without CKD, defined as an estimated glomerular filtration rate <60 ml/min/1.73 m 2 , were categorized into those with single-vessel disease and those with multivessel disease with or without CTO. The primary outcomes were the incidence of 30-day and 1-year mortality. Among 1,873 consecutive patients with STEMIs included between 2006 and 2011, 336 (18%) had CKD. The prevalence of CTO in a non-infarct-related artery was 13% in patients with CKD compared with 7% in those without CKD (p = 0.0003). There was a significant interaction between CKD and CTO on 30-day mortality (p = 0.018) and 1-year mortality (p = 0.013). Independent predictors of late mortality in patients with CKD were previous myocardial infarction (hazard ratio [HR] 1.71, 95% confidence interval [CI] 1.01 to 2.79), age >75 years (HR 1.86, 95% CI 1.19 to 2.95), a left ventricular ejection fraction after primary PCI <40% (HR 2.20, 95% CI 1.36 to 3.63), left main culprit artery (HR 4.46, 95% CI 1.64 to 10.25), and shock (HR 7.44, 95% CI 4.56 to 12.31), but multivessel disease with CTO was not a predictor. In contrast, multivessel disease with CTO was an independent predictor of mortality in patients without CKD (HR 3.30, 95% CI 1.70 to 6.17). In conclusion, in patients with STEMIs who underwent primary PCI, with preexisting CKD, the prevalence of CTO in a non-infarct-related artery was twice as great. In these patients, the clinical impact of CTO seems to be overshadowed by the presence of CKD.
Decreasing renal function is associated with an increasing prevalence of cardiovascular disease. In subjects without known coronary artery disease, the risk for myocardial infarction (MI) is similar between those with chronic kidney disease (CKD) and those with diabetes mellitus. CKD is also a major risk factor for cardiovascular complications and mortality after MI. The excess mortality in this population appears to be mediated through arrhythmias, adverse hemodynamic events, and lower use of mortality-reducing therapy. Multivessel disease (MVD) is also more prevalent in patients admitted for acute MI with CKD, and these patients frequently have had previous MIs. A chronic total occlusion (CTO) could be considered the hallmark of an old MI, and recent studies have suggested that the effect of MVD on mortality in patients with ST-segment elevation MIs (STEMIs) was essentially due to the presence of a CTO in an artery other than the infarct-related artery (IRA). This observation was found to be also true in high-risk patients with STEMIs with diabetes. However, the impact of CTO in a non-IRA according to baseline renal function has not been previously assessed. This could directly affect the choice, type, and timing of revascularization in patients with MVD and CTO after initial primary percutaneous coronary intervention (PCI). Our objectives were to study the prevalence and impact of CTO in a non-IRA on long-term mortality in unselected patients with STEMIs according to baseline renal function.
Methods
The study population included all 2,144 consecutive patients with STEMIs who were referred to our center for primary PCI <12 hours after symptom onset from January 2006 to January 2011. All patients provided written informed consent to be part of a STEMI registry. The registry was approved by the local institutional review board. The inclusion criteria were chest pain lasting >30 minutes, ST-segment elevation ≥1 mm in ≥2 adjacent electrocardiographic leads, new left bundle branch block, and true posterior MI. All patients with STEMI were pretreated with aspirin, clopidogrel, and heparin. Cardiac catheterization was performed using the radial approach with 6Fr guiding catheters in most patients. Adjunctive pharmacotherapy such as bivalirudin or glycoprotein IIb/IIIa inhibitors was left to the operator’s discretion. If the coronary anatomy was suitable for PCI, the procedure was performed using standard techniques. All angiograms were prospectively reviewed by 2 independent readers, with discrepancies resolved by a third reader and consensus. For the purposes of this study, MVD was defined by visual assessment as any stenosis >70% of the coronary luminal diameter in ≥1 of the non-IRAs or left main stenosis ≥50%. A CTO was defined as a total occlusion in a non-IRA without anterograde flow or with anterograde or retrograde filling through collateral vessels. Furthermore, only CTOs of major epicardial coronary arteries or major side branches ≥2 mm in diameter and judged to be (1) amenable to PCI and (2) subtending significant myocardial territory were evaluated. The differentiation between CTO and subacute or acute occlusions was made on the basis of the morphology of the occlusion (absence of fresh thrombus, presence of bridging, epicardial or septal collateral vessels) and a possible history of MI. Myocardial blush grade was assessed for the IRA as previously described.
Baseline serum creatinine on hospital admission was determined using Jaffe’s method and standardized to isotope dilution mass spectrometry. The Chronic Kidney Disease Epidemiology Collaboration equation was used to calculate estimated glomerular filtration rate (eGFR). This equation is more accurate than the Modification of Diet in Renal Disease (MDRD) study equation, especially at higher values of glomerular filtration rate, and has demonstrated better predictive values for clinical outcomes in the general population and in patients with acute MIs. The eGFR was categorized as ≥60 or <60 ml/min/1.73m 2 , corresponding to stages 1 and 2 and stages 3 to 5 of CKD, respectively. An eGFR <60 ml/min/1.73 m 2 was defined as indicating CKD.
Patients with previous coronary artery bypass grafts (n = 73) as well as those with STEMIs without significant coronary lesions (n = 51) were excluded from the study. Baseline in-hospital data for the 2,020 included patients were obtained from a computerized database of prospectively recorded demographic, clinical, and procedural information. The left ventricular ejection fraction was assessed <3 days after the index event using left ventricular angiography, echocardiography, or cardiac magnetic resonance imaging. Clinical follow-up information was obtained from referring physicians or by phone contact with patients. Finally, information on vital status at 1 year of those who were lost to follow-up was collected from the Quebec death registry Directeur de l’Etat Civil service counter. The primary outcomes were 30-day and 1-year all-cause mortality.
Categorical variables are expressed as numbers and percentages and continuous variables as mean ± SD or median (interquartile range). Baseline and procedural characteristics were compared using Fischer’s exact test for categorical variables and Student’s t test or Wilcoxon’s test for continuous variables. Survival curves were constructed using Kaplan-Meier techniques, and comparisons between groups were done using the log-rank test. The Cox proportional-hazards model was used to identify the independent predictors of 30-day and 1-year mortality in each eGFR category. Stepwise selection was used to identify potential predictors in each eGFR category, which were entered into the model at p <0.10 and retained at p <0.10. A p value <0.05 was considered statistically significant. Candidates for adjustment included age, gender, weight, diabetes, hypertension, current smoking, hyperlipidemia, history of angina, previous MI, door-to-balloon time, previous PCI, shock on hospital admission, MVD without CTO, MVD with CTO, IRA left main coronary artery, IRA left anterior descending coronary artery, a left ventricular ejection fraction <40%, Thrombolysis In Myocardial Infarction (TIMI) flow grade 0 or 1 and myocardial blush grade 0 or 1 after PCI, and peak creatine kinase-MB. Analysis was performed to test the interaction for 30-day and 1-year mortality between CKD and CTO. Calculations and statistical analysis were performed using JMP version 9.0.0 (SAS Institute Inc., Cary, North Carolina).
Results
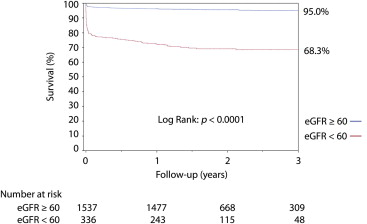
A total of 1,873 patients with STEMIs with baseline serum creatinine levels available at the time of hospital admission were included in this analysis. The distribution of eGFR was wide, with a mean value of 79 ± 21 ml/min/1.73 m 2 . Clinical follow-up was complete for 100% of patients at 1 year (median follow-up 574 days, range 389 to 959). Among the 1,873 patients, 1,537 (82%) had eGFRs ≥60 ml/min/1.73 m 2 , and 336 (18%) had eGFRs <60 ml/min/1.73 m 2 (i.e., they had CKD; Table 1 ). The prevalence of a CTO in a non-IRA was almost twice as high in patients with CKD compared with those with eGFRs ≥60 ml/min/1.73 m 2 (7% vs 13.4%, respectively, p = 0.0003). Patients with CKD exhibited longer door-to-balloon times (p <0.0001). Abnormal epicardial or myocardial flow in the IRA (TIMI flow grade 0 or 1 and myocardial blush grade 0 or 1) after primary PCI occurred more frequently in patients with CKD (p <0.0001). At 30-day follow-up, patients with CKD were less frequently treated with clopidogrel (89%) compared with those with eGFRs ≥60 ml/min/1.73 m 2 (94%) (p = 0.03), without any other significant difference regarding the use of aspirin, β blockers, angiotensin-converting enzyme inhibitors, or statins (data not shown). An eGFR <60 ml/min/1.73 m 2 was associated with higher mortality ( Figure 1 ). Unadjusted Kaplan-Meier estimates for 1-year mortality were 5% in patients with eGFRs ≥60 ml/min/1.73 m 2 and 32% in patients with CKD (p <0.0001). After adjustment, an eGFR <60 ml/min/1.73 m 2 was associated with a hazard ratio (HR) for late death of 3.1 (95% confidence interval [CI] 2.07 to 4.83, p <0.0001).
| Variable | eGFR ≥60 ml/min/1.73 m 2 (n = 1,537 [82%]) | eGFR <60 ml/min/1.73 m 2 (n = 336 [18%]) | p Value |
|---|---|---|---|
| Age (yrs) | 59 ± 11 | 72 ± 12 | <0.0001 |
| Women | 309 (20%) | 132 (39%) | <0.0001 |
| Diabetes mellitus treated | 164 (11%) | 67 (20%) | <0.0001 |
| Hypertension treated | 595 (39%) | 209 (62%) | <0.0001 |
| Current smoking | 703 (46%) | 88 (26%) | <0.0001 |
| Dyslipidemia treated | 606 (39%) | 158 (47%) | 0.012 |
| History of angina | 198 (13%) | 63 (19%) | 0.007 |
| Previous MI | 168 (11%) | 61 (18%) | 0.0004 |
| Previous PCI | 137 (9%) | 44 (14%) | 0.018 |
| Cardiogenic shock | 70 (5%) | 70 (21%) | <0.0001 |
| Radial access | 1,479 (96%) | 285 (85%) | <0.0001 |
| Single-vessel disease | 1,117 (73%) | 183 (54%) | <0.0001 |
| MVD without CTO | 311 (20%) | 108 (32%) | <0.0001 |
| MVD with CTO | 109 (7%) | 45 (13%) | 0.0003 |
| IRA | |||
| Left main coronary artery | 5 (0.3%) | 7 (2%) | 0.002 |
| Left anterior descending coronary artery | 624 (41%) | 130 (39%) | 0.54 |
| Right coronary artery | 690 (45%) | 160 (48%) | 0.36 |
| Circumflex coronary artery | 218 (14%) | 39 (12%) | 0.25 |
| TIMI flow grade 0 before PCI | 911 (59%) | 176 (53%) | 0.028 |
| Symptom onset to first balloon inflation (h) | 4.1 (2.5–7.6) | 4.3 (2.7–7.7) | 0.19 |
| Door to first balloon inflation (minutes) | 37 (28–65) | 45 (31–74) | <0.0001 |
| Use of glycoprotein IIb/IIIa inhibitors | 1,127 (73%) | 183 (54%) | <0.0001 |
| TIMI flow grade 0 or 1 after PCI | 28 (2%) | 21 (6%) | <0.0001 |
| Angiographic success | 1,211 (79%) | 202 (68%) | <0.0001 |
| Myocardial blush grade 0 or 1 | 111 (8%) | 53 (17%) | <0.0001 |
| Contrast volume (ml) | 180 (140–225) | 175 (130–210) | 0.11 |
| Stent implanted | 1,493 (97) | 321 (95%) | 0.11 |
| Coronary artery bypass graft ≤30 days | 29 (2%) | 8 (3%) | 0.52 |
| PCI after index procedure ≤30 days | 98 (6%) | 22 (6%) | 0.90 |
| Left ventricular ejection fraction (%) | 52 ± 13 | 46 ± 16 | <0.0001 |
| Peak creatine kinase-MB (μg/L) | 145 (62–276) | 153 (71–284) | 0.45 |
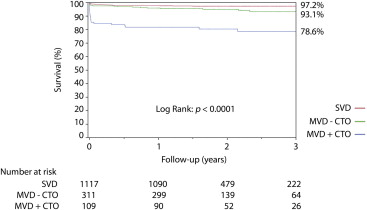
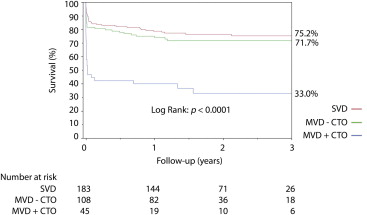
Figures 2 and 3 show the cumulative survival curves by eGFR category according to the extent of coronary artery disease. In patients with CKD, late mortality was significantly worse in patients with MVD and CTO (77.0%) compared with those with MVD but no CTO (28.3%) or with single-vessel disease (24.8%) (p <0.0001). In patients without CKD, the late survival rate was also significantly worse in patients with CTO (21.4%) compared with those with MVD without CTO (6.8%) or with single-vessel disease (2.8%) (p <0.0001; Figure 2 ). Importantly, there was a significant interaction between CKD and CTO on 30-day mortality (HR 1.30, 95% CI 1.05 to 1.61, p = 0.018) and on 1-year mortality (HR 1.28, 95% CI 1.05 to 1.55, p = 0.013).
Tables 2 and 3 show the independent predictors of 30-day and 1-year mortality in each eGFR category. In patients with CKD, the presence of CTO in a non-IRA was not an independent predictor of 30-day or 1-year mortality. In contrast, CTO was found to be an independent clinical predictor of 30-day and 1-year mortality in patients with eGFRs ≥60 ml/min/1.73 m 2 , with HRs of 3.76 (95% CI 1.76 to 7.80, p = 0.0008) and 3.30 (95% CI 1.70 to 6.17, p = 0.0006), respectively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


