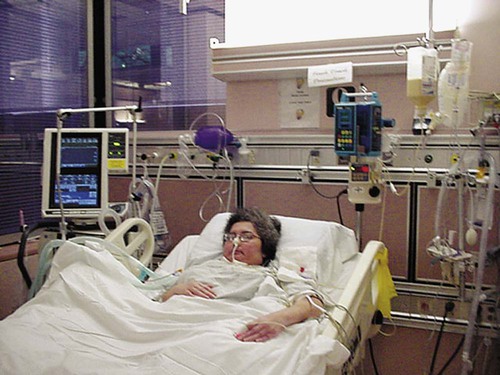
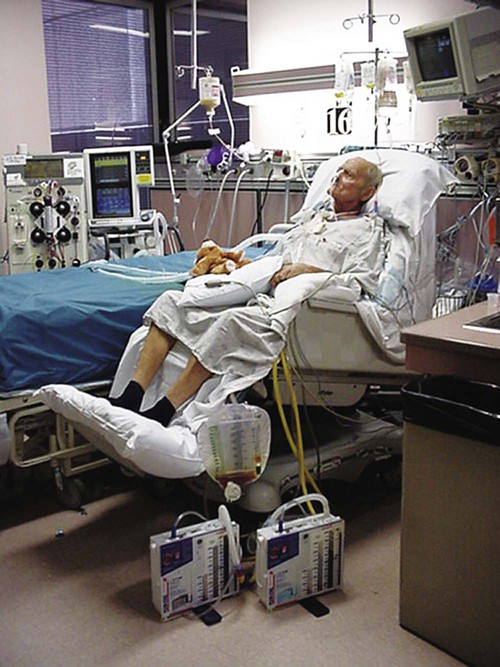
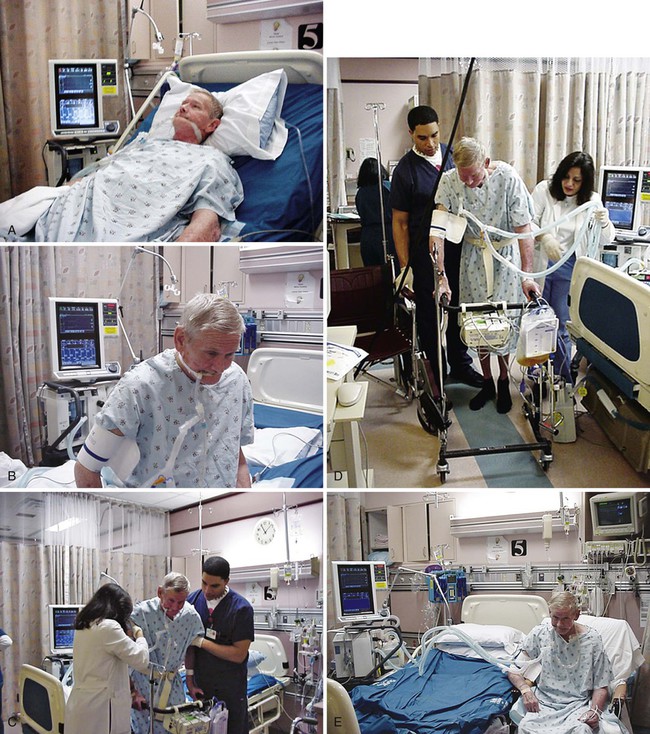
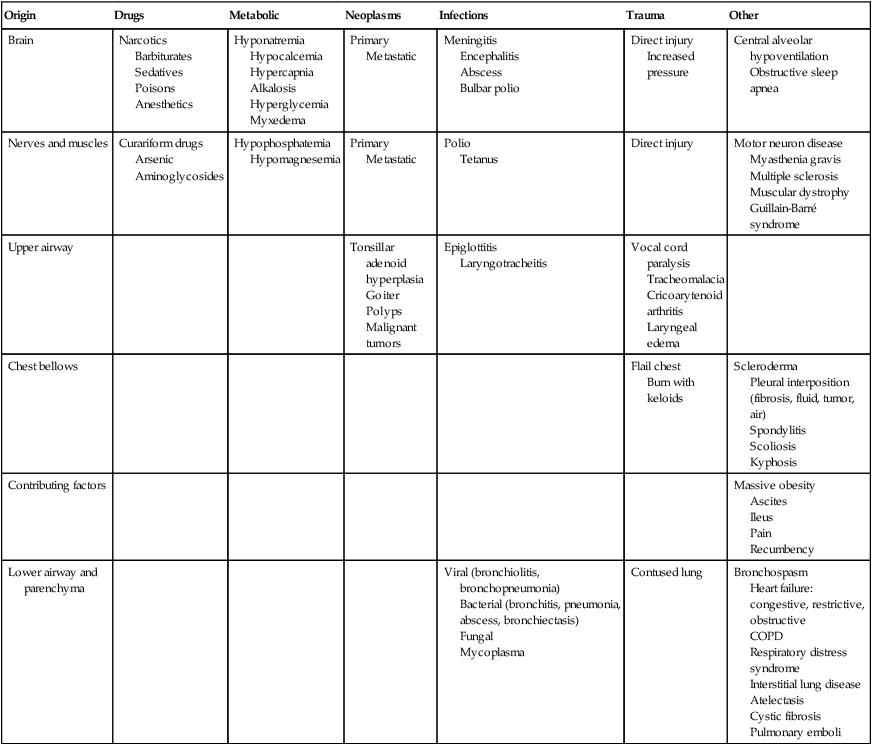
The principles of physical therapy management that are described are not treatment prescriptions. Rather, each patient must be assessed and treated individually, taking into consideration all factors that contribute to impaired oxygen transport (i.e., recumbency, restricted mobility, extrinsic factors related to the patient’s care, intrinsic factors related to the patient, and the underlying pathophysiology) (see Chapter 2). Pulmonary failure reflects a gas exchange defect or defect of the ventilatory pump. Table 34-1 shows the classification of pulmonary failure by specific causes and the mechanisms involved. Some common predisposing conditions include primary cardiovascular and pulmonary conditions (e.g., chronic lung disease, overwhelming pneumonia, and myocardial infarction [MI]) and cardiovascular and pulmonary conditions that are secondary to other conditions (e.g., motor neuron diseases, spinal cord injury, stroke, and muscular dystrophy) (see Chapter 35). The oxygen and carbon dioxide tensions that have been used to define failure are variable because they depend on factors such as premorbid status, general health, age, prior blood gas profile, and the time frame for the development of failure. Arterial blood gases and pH are essential in the assessment of cardiovascular and pulmonary failure, which is usually diagnosed when the PaO2 falls below 50 to 60 mm Hg and the PaCO2 rises above 50 mm Hg.1 Table 34-1 Classification of Respiratory Failure by Cause and Mechanism From Civettia, J.M. Taylor, R.W. & Kirby, R.R (1988). Critical care, Philadelphia: JB Lippincott. Obstructive lung disease can result in ventilatory failure and admission of the patient to the ICU, or it can complicate management if the patient is admitted for other reasons.2 If conservative management fails or is unlikely to improve critically impaired oxygen transport and gas exchange and to adequately remove copious and tenacious secretions, intubation and mechanical ventilation are indicated (see Chapter 43). Complicating factors include impaired oxygen delivery, polycythemia, impaired respiratory mechanics secondary to lung damage and increased time constants impairing optimal inhalation and exhalation, flattened hemidiaphragms, rigid barrel-shaped chest wall, increased accessory muscle use and work of breathing, reduced diffusing capacity, impaired mucociliary transport, secretion accumulation, ineffective cough mechanism, increased oxygen consumption, increased work of the heart, and general debility and weakness. The goal of intubation and mechanical ventilation is to support breathing by providing an airway and adequate alveolar ventilation and is based on arterial blood gas analysis. A tidal volume and a respiratory rate that provide satisfactory blood gas and pH values are established and maintained unless the clinical condition changes. The precise regulation of mechanical ventilation helps to restore adequate blood gases and cardiovascular and pulmonary function, reduce the work of breathing, rest fatigued ventilatory muscles, and provide an optimal fraction of inspired oxygen (FIO2) and humidification (Figure 34-1). Positive end-expiratory pressure (PEEP) is useful in promoting greater opportunity for gas exchange at end-expiration in mechanically ventilated patients. Venous return, myocardial perfusion, and cardiac output, however, may be impaired during positive-pressure ventilation with PEEP administration.3 Excessive stimulation to cough in these ventilated patients should be avoided because this accentuates the cardiovascular side effects of PEEP. Continuous positive airway pressure (CPAP) can maintain airway patency during spontaneous ventilation. This mode of ventilation, however, seems to be preferred in children, whereas PEEP is used more commonly in adults. Suctioning can be performed frequently in a patient with an artificial airway and is less traumatic. Patients should be suctioned only as indicated because this procedure can produce significant desaturation (up to 60%), particularly in the ventilated patient.4 Administration of 100% oxygen for 3 minutes before and after suctioning (i.e., hyperoxygenation) minimizes this desaturation effect. This can be accomplished by manual bagging before treatment (i.e., manual hyperventilation) or by presetting of the mechanical ventilator. Risk of aspiration of gastric contents is reduced by the use of a nasogastric tube. A common cause of acute respiratory failure is advanced chronic airflow limitation.5 The pathophysiological deficits include significant loss of alveolar tissue, increased compliance of alveolar tissue, hyperinflated chest wall, impaired respiratory mechanics, flattened hemidiaphragms, impaired breathing efficiency, and reduced diffusing capacity. Proportional changes in lung volumes and capacities in patients with chronic airway limitation compared with healthy persons are presented in Figure 9-5. The primary abnormalities are significantly increased residual volume and inspiratory reserve volume and hence total lung capacity. Failure of oxygen transport ensues secondary to ventilation and perfusion mismatch, ventilatory muscle fatigue, reactive pulmonary hypertension, and right ventricular failure. Correcting the complications of respiratory failure, however, is often more problematic than treating the specific cause. Hypoxemia and hypercapnia are often present. Hypoxemia is usually improved with supplemental oxygen in the absence of significant diffusion defect or shunt. Cardiovascular complications are among the most prevalent observed in ventilatory failure. Marked hypercapnia (increased arterial PCO2) with acidemia (reduced pH) can produce extreme vasodilatation and hypotension resulting from the local action on blood vessels.2 Mild hypercapnia can produce reflex vasoconstriction and hypertension. Occasionally, systemic hypertension is observed during weaning from the ventilator with the presence of a moderate degree of hypercapnia. End-stage respiratory failure results in a progressive increase in airway resistance, work of breathing, oxygen consumption, and carbon dioxide production. In areas of bronchial obstruction, marked alveolar hypoventilation results and ventilation and perfusion are severely mismatched. Hypoxemia and respiratory acidosis produce reactive pulmonary hypertension and further ventilatory failure. Profound carbon dioxide retention, refractory hypoxemia, and respiratory acidemia may terminate in a fatal dysrhythmia.6 Of increasing interest in recent years has been the experience of illness and its contribution to disability. After their first episode of respiratory failure, patients with chronic obstructive pulmonary disease (COPD) report worse cognitive function and overall health status. After several months, however, these conditions may return to levels reported by individuals with similar disease severity who are being conservatively managed and have had no ICU admission.7 The Nottingham Health Profile and Mini-Mental State Examination can be useful tools for ongoing assessment of perceived health and cognition. The principles of management of acute respiratory failure secondary to an exacerbation of COPD are based on interventions that will enhance oxygen transport (i.e., oxygen delivery, oxygen consumption, and oxygen extraction) and facilitate carbon dioxide removal. Thus the steps of the oxygen transport pathway that have been identified as impaired or threatened by the patient’s condition (i.e., restricted mobility, recumbency, and extrinsic and intrinsic factors in addition to the underlying pathophysiology) (see Chapter 2) are the focus of treatment.8 On the basis of a detailed analysis of these factors, treatments are selected, prioritized, and applied to optimize the steps in the pathway that are affected. These may include treatments to maximize the patency of the airways, increase alveolar ventilation, facilitate mucociliary transport, facilitate airway clearance, optimize the mechanical position of the diaphragm, optimize ventilation and perfusion matching, optimize pH, eliminate carbon dioxide, optimize peripheral circulation and tissue perfusion, and reduce the work of breathing and of the heart. To optimize oxygen transport, the primary goals of physical therapy management include the following: 1. Improve or maintain arterial oxygen tension (PaO2) or prevent its deterioration 2. Improve or maintain arterial oxygen saturation (SaO2) or prevent its deterioration 3. Improve or maintain arterial carbon dioxide levels (PaCO2) and pH or prevent their deterioration 4. Optimize oxygen delivery and oxygen consumption relationship Mobilization and ambulation are required for normal physiological functioning of the human body (i.e., to stimulate exercise stress and gravitational stress and thereby optimize oxygen transport).9,10 Although patients in the ICU are encouraged to move, exercise, sit up, stand, sit in chairs, take a few steps, and in some circumstances ambulate across the unit even if they are ventilated, therapeutic mobilization is prescribed to exploit its acute effects, long-term cumulative effects, and preventive effects (see Chapter 18). These effects are physiologically distinct and need to be prescribed specifically to address each patient’s problems. With the monitoring capability in the ICU to ensure safety, patients who are critically ill can move and be moved within safe as well as therapeutic limits. Given that cardiovascular and pulmonary physical therapy is among those activities that are the most metabolically demanding for patients in the ICU,11–14 the patient’s capacity to meet a given increase in oxygen demand must be determined before treatment. Even though cardiovascular and pulmonary physical therapy stresses the oxygen transport system as a means of improving the function and efficiency of this system, unnecessary or excessive energy expenditure is undesirable and thus should be minimized. Interventions that can minimize oxygen demand include relaxation, judicious body positioning to facilitate oxygen transport, coordination of treatments with other interventions, scheduling of treatments at appropriate times, timing of treatments with medications so the maximal effect is achieved, pain control, and coordination of treatments with peak energy periods and around rest periods. Mobilization and exercise are at the top of the hierarchy of physiological treatments in the management of patients in the ICU15–17; therefore the potent and direct effects of these interventions are exploited first (see Chapter 17). Being moved to (passive) or moving into (active) the upright position promotes improved cardiovascular and pulmonary function and gas exchange. A supported chair should be available beside every ICU bed to provide greater opportunity for the patient to be upright when out of bed. The benefits of the upright sitting position are different from those of sitting propped up in bed. Stretcher chairs are particularly useful for patients who are unable to bear weight (Figure 34-2). There are also beds that are designed to position patients sitting. Even minimal ability of the patient to assist with his or her bed mobility and transferring must be exploited, even if the maneuver takes several minutes and multiple assistants. These minimal efforts must be exploited, or the patient’s oxygen transport system will decondition further, which also will reduce the patient’s tolerance for being mobilized. What justifies this time and personnel cost is the greater therapeutic outcome that can be expected compared with passive interventions. The ultimate goal is enhanced recovery, reduced discomfort, reduced morbidity and mortality, and reduced ICU and hospital stay. Significant benefit can be gained from standing the ventilated patient, provided there are no absolute contraindications to being upright in terms of cardiovascular and pulmonary function, neuromuscular and musculoskeletal status, and skin integrity. Ambulating the patient who is ventilated is the priority whenever possible.18–20 The potential risks, however, must be recognized. In a prospective study, Bailey and colleagues (2007)21 documented a total of 1449 “activity events” in 103 patients with respiratory failure. These activity events included 233 (16%) instances of sitting on the bed, 454 (31%) instances of sitting in a chair, and 762 (53%) instances of ambulating. In patients with an endotracheal tube, there were 593 activity events reported, of which 249 (42%) were ambulation. Fewer than 1% adverse activity-related events were reported (including fall to the knees without injury, feeding tube removal, systolic blood pressure greater than 200 mm Hg, systolic blood pressure less than 90 mm Hg, and desaturation less than 80%). No patient was extubated during an activity event. Thus activity and mobilization are feasible and safe in patients with respiratory failure. Of note, the majority of survivors (69%) were able to ambulate over 100 feet at discharge from the ICU. These findings have been corroborated by others.22,23 Mobilization and exercise must be prescribed specifically, however, to ensure that the exercise stimulus is therapeutic (i.e., provides an adequate stressor to the oxygen transport pathway yet is not hazardous). Standing and walking even a few steps can be extremely strenuous with respect to oxygen demand for the patient in the ICU. Such activities need to be introduced gradually and with continuous monitoring to ensure that the patient does not exceed the prescribed therapeutic intensity needed to maximize oxygen transport. Standing and walking are coordinated with other aspects of the patient’s care and should be carried out in several stages (Figure 34-3). Monitoring of the electrocardiogram (ECG) and arterial saturation of the critically ill patient while he or she performs activities such as standing or walking cannot be overemphasized. In anticipation of an increased workload, ventilatory parameters for ventilated patients may require adjusting. A greater concentration of oxygen should be delivered for at least 3 to 5 minutes before the activity and continued afterward for 10 minutes or so until the patient has recovered from the increased exertion and the heart rate and blood pressure have returned to within 5% to 10% of baseline values.
Intensive Care Management of Individuals with Primary Cardiovascular and Pulmonary Dysfunction
Cardiovascular and Pulmonary Failure
Pathophysiology and Medical Management
Origin
Drugs
Metabolic
Neoplasms
Infections
Trauma
Other
Brain
Narcotics
Barbiturates
Sedatives
Poisons
Anesthetics
Hyponatremia
Hypocalcemia
Hypercapnia
Alkalosis
Hyperglycemia
Myxedema
Primary
Metastatic
Meningitis
Encephalitis
Abscess
Bulbar polio
Direct injury
Increased pressure
Central alveolar hypoventilation
Obstructive sleep apnea
Nerves and muscles
Curariform drugs
Arsenic
Aminoglycosides
Hypophosphatemia
Hypomagnesemia
Primary
Metastatic
Polio
Tetanus
Direct injury
Motor neuron disease
Myasthenia gravis
Multiple sclerosis
Muscular dystrophy
Guillain-Barré syndrome
Upper airway
Tonsillar adenoid hyperplasia
Goiter
Polyps
Malignant tumors
Epiglottitis
Laryngotracheitis
Vocal cord paralysis
Tracheomalacia
Cricoarytenoid arthritis
Laryngeal edema
Chest bellows
Flail chest
Burn with keloids
Scleroderma
Pleural interposition (fibrosis, fluid, tumor, air)
Spondylitis
Scoliosis
Kyphosis
Contributing factors
Massive obesity
Ascites
Ileus
Pain
Recumbency
Lower airway and parenchyma
Viral (bronchiolitis, bronchopneumonia)
Bacterial (bronchitis, pneumonia, abscess, bronchiectasis)
Fungal
Mycoplasma
Contused lung
Bronchospasm
Heart failure: congestive, restrictive, obstructive
COPD
Respiratory distress syndrome
Interstitial lung disease
Atelectasis
Cystic fibrosis
Pulmonary emboli
Obstructive Lung Disease
Pathophysiology and Medical Management
Principles of Physical Therapy Management
Mobilization: Special Considerations
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Intensive Care Management of Individuals with Primary Cardiovascular and Pulmonary Dysfunction