Statins are recommended for prevention of progression of cardiovascular disease after percutaneous coronary intervention (PCI). Although high-dose highly efficient statins are recommended, especially in high-risk patients, clinical data are scarce and further investigation in “real-world” settings is needed. One thousand five hundred twenty-eight consecutive patients, who underwent PCI for acute coronary syndrome, were included in a prospective registry from January 2003 to January 2011. In post hoc analysis, cardiovascular risk factors, co-morbidities, and circulating lipid parameters at the time of intervention were evaluated. As a primary end point, all-cause mortality after a follow-up period of 3 months was investigated. Results were compared between patients receiving high-dose highly effective statins (atorvastatin 80 mg or rosuvastatin 20 mg) versus patients receiving low-dose statins or who were without lipid-lowering therapy at the time of discharge. Nine hundred twenty-six patients (60.6%) received high-dose atorvastatin or rosuvastatin and 602 patients (39.4%) received low-dose statin therapy or were not on statins at discharge. Eight patients (0.9%) receiving high-dose statin therapy and 21 patients (3.5%) taking low-dose statins or no statins at discharge died during the 3-month follow-up (hazard ratio 0.244, 95% confidence interval 0.108 to 0.551, p = 0.001). After propensity score adjustment the results remained significant (adjusted hazard ratio for high-dose statins 0.405, 95% confidence interval 0.176 to 0.931, p = 0.033). In conclusion, in this single-center series of 1,528 real-world patients undergoing PCI for acute coronary syndrome, a significant reduction in short-term all-cause mortality could be demonstrated in patients receiving high-dose highly efficient statins compared with patients receiving low-dose statins or no lipid-lowering therapy.
Dyslipidemia is a very important risk factor for cardiovascular events, and lowering of low-density lipoprotein cholesterol (LDL-C) with statins is a standard treatment in patients with coronary heart disease according to the current guidelines. Many clinical trials demonstrated that statins can reduce both atherogenic lipoproteins and cardiovascular event rates compared with placebo, and intensive statin therapy is more effective than standard therapy. However, the efficacy of different statins varies significantly and the effect of these differences in clinical outcome is still under debate. The optimal type and dose of statins in patients with acute coronary syndrome (ACS) in clinical practice remains unclear and there are scarce “real-world” data from observational studies. The aim of this study was to investigate the effect of high-dose highly efficient statins on short-term mortality in patients with ACS undergoing percutaneous coronary intervention (PCI) and stent implantation in a real-world setting.
Methods
In total, 1,528 consecutive patients, who underwent PCI for ACS, were included in a prospective registry from January 2003 to January 2011. Eighty-one patients (52.4%) had an ST elevation myocardial infarction (STEMI) and 727 (47.6%) had a non-STEMI at presentation. In post hoc analysis, cardiovascular risk factors, co-morbidities, and basal circulating lipid variables (total cholesterol, LDL-C, high-density lipoprotein cholesterol, and triglycerides) at the time of the index event were evaluated. Results were compared between patients receiving high-dose highly effective statins (atorvastatin 80 mg or rosuvastatin 20 mg) versus patients receiving low-dose statins or without lipid-lowering therapy at discharge. It was at the discretion of the treating physician to prescribe a statin and to choose the dose.
Before stent implantation, all patients were treated with aspirin (100 mg/day after a loading dose of intravenous 250 mg) and clopidogrel or prasugrel. After the procedure, all patients received dual antiplatelet therapy according to the then existing guidelines, that is, for 9 to 12 months.
The study was performed in accordance with the Declaration of Helsinki and approved by the local ethics committee (EK 10-046-VK_NZ).
As the primary end point of this study, all-cause mortality after a follow-up period of 3 months (the period in which the patients were documented to be consistently on therapy) was evaluated. All patients completed the follow-up (100% follow-up rate). Mortality data for all patients were obtained from Statistics Austria. (Statistics Austria is an independent and non–profit-making federal institution under public law and supports scientific services).
Continuous variables are described as mean ± SD unless stated otherwise. Categorical variables were compared using the Pearson chi-square test, and Student t test was used, if appropriate, to compare continuous variables. Univariate Cox regression analysis was performed to estimate the survival of patients with high-dose statins and low dose or without statins. As this is a retrospective analysis of nonrandomized data and patients with high-dose statins and low dose or without statins showed significant differences in important clinical aspects, we performed propensity score analysis for each patient to estimate the likelihood of receiving high-dose statins. The following patient characteristics and angiographic data were used for the calculation of the score: age, gender, history of hypertension, hypercholesterolemia, diabetes mellitus, smoking, renal dysfunction, peripheral arterial disease, malignant tumor, previous PCI, coronary bypass surgery, myocardial infarction, heart failure, and type of the stent (drug-eluting or bare-metal stent), size of the stent, and length of the stent implanted. The propensity score was then included as a covariate in the Cox regression analysis. We report both unadjusted and propensity-score adjusted hazard ratios (HRs) with 95% confidence intervals (CIs). All statistical tests were 2-tailed and statistical significance was accepted if the p value was <0.05. The Software Package for Social Sciences (SPSS Inc., Chicago, Illinois) was used for all statistical calculations except for the estimation of the propensity score (Stata; StataCorp, College Station, Texas).
Results
Nine hundred twenty-six patients (60.6%) received high-dose atorvastatin (91.9%) or rosuvastatin (8.1%) and 602 patients (39.4%) received low-dose statin therapy with lower doses of atorvastatin, rosuvastatin, or different statins, or were not on statins at discharge due to normal cholesterol levels. Their baseline mean plasma levels of lipid variables are listed in Table 1 . Sixty-six patients (7.1%) with high-dose statins and 44 patients (9.1%) with low-dose statins or without statin (p = 0.23) were on therapy with a statin on admission.
| Baseline Lipid Levels | High-Dose Statin (n = 926) | Low Dose or No Statin (n = 602) | p Value |
|---|---|---|---|
| Total cholesterol (mg/dl) | 197 ± 60 | 172 ± 45 | <0.001 |
| LDL-C (mg/dl) | 124 ± 53 | 98 ± 84 | <0.001 |
| High-density lipoprotein cholesterol (mg/dl) | 43 ± 15 | 43 ± 16 | 0.78 |
| Triglycerides (mg/dl) | 155 ± 100 | 150 ± 100 | 0.23 |
Of a series of clinical characteristics, the following were significantly different between the study groups: age, STEMI at presentation, current smoking, cerebrovascular disease, peripheral arterial disease, history of myocardial infarction, history of coronary artery bypass grafting and renal dysfunction, drug-eluting stent implantation, and maximal stent length, thus demonstrating an overall higher risk for atherothrombotic events and mortality in the low dose or without statin arm. Gender, arterial hypertension, diabetes mellitus, previous PCI, heart failure, 3-vessel disease, and maximal stent diameter were not different between the groups ( Table 2 ).
| Variable | High-Dose Statin (n = 926) | Low Dose or No Statin (n = 602) | p Value |
|---|---|---|---|
| Age (yrs) | 60.81 ± 12.92 | 65.31 ± 13.28 | <0.001 |
| Men | 629 (68.4) | 387 (64.5) | 0.11 |
| Arterial hypertension | 688 (74.3) | 454 (75.4) | 0.62 |
| Diabetes mellitus | 203 (21.9) | 147 (24.4) | 0.25 |
| Heart failure | 57 (8.8) | 45 (8.2) | 0.70 |
| STEMI | 537 (57.8) | 218 (44.9) | <0.001 |
| Renal dysfunction | 180 (20.2) | 192 (32.5) | 0.001 |
| Previous myocardial infarction | 106 (11.4) | 91 (15.1) | 0.037 |
| Previous PCI | 98 (10.6) | 76 (12.6) | 0.22 |
| Previous coronary arterial bypass graft | 17 (1.8) | 21 (3.5) | 0.04 |
| Smoker | 281 (43.2) | 172 (31.2) | <0.001 |
| Cerebrovascular disease | 35 (3.8) | 45 (7.5) | 0.002 |
| Peripheral arterial disease | 19 (2.1) | 38 (6.4) | <0.001 |
| Drug-eluting stent implantation | 215 (33.3) | 122 (22.3) | <0.001 |
| 3-Vessel coronary disease | 231 (24.9) | 144 (23.9) | 0.64 |
| Maximal stent length | 19.77 ± 6.35 | 18.46 ± 5.95 | 0.007 |
| Maximal stent diameter | 3.06 ± 0.36 | 3.11 ± 0.41 | 0.18 |
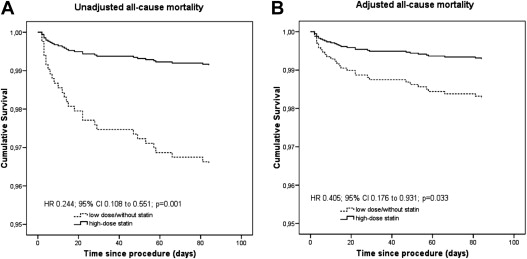
Eight patients (0.9%) receiving high-dose statin therapy and 21 patients (3.5%) taking low-dose statins or without statins at discharge died during the 3-month follow-up (HR 0.244, 95% CI 0.108 to 0.551, p = 0.001). After adjustment using propensity score analysis the results remained significant (adjusted HR for high-dose statins 0.405, 95% CI 0.176 to 0.931, p = 0.033; Figure 1 ).
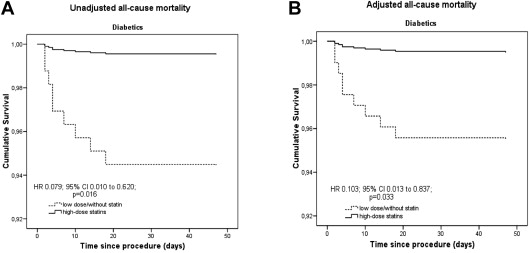
Diabetics, who were treated with high-dose statins, exhibited a significantly lower all-cause mortality than diabetics of the low dose or without statin group (0.5% vs 6.1%, HR 0.079, 95% CI 0.010 to 0.620, p = 0.016; adjusted HR for high-dose statins 0.103, 95% CI 0.013 to 0.837, p = 0.033; Figure 2 ), whereas there was no significant difference for nondiabetic patients after adjustment with the propensity score (1% vs 2.6%, HR 0.364, 95% CI 0.143 to 0.923, p = 0.033; adjusted HR for high-dose statins 0.649, 95% CI 0.251 to 1.678, p = 0.372; Figure 3 ).
Gender had no influence on mortality within the treatment groups (men, high dose: 0.8% vs low dose or no statin: 3.1%, HR 0.252, 95% CI 0.089 to 0.716, p = 0.01; adjusted HR for high-dose statin 0.411, 95% CI 0.141 to 1.20, p = 0.10; women, high dose: 1% vs low dose or no statin: 4.2%, HR 0.239, 95% CI 0.065 to 0.881, p = 0.032; adjusted HR for high-dose statin 0.398, 95% CI 0.106 to 1.498, p = 0.173).
Patients with renal dysfunction and an estimated glomerular filtration rate of <60 ml/min/1.75 m 2 had a benefit with high-dose statin compared with low dose or no statin (high dose: 2.2% vs low dose or no statin: 7.8%, HR 0.017, 95% CI 0.088 to 0.786, p = 0.017; adjusted HR for high-dose statin 0.318, 95% CI 0.105 to 0.962, p = 0.043). This benefit could not be demonstrated in patients with estimated glomerular filtration rate >60 ml/min/1.75 m 2 (high dose: 0.6% vs low dose or no statin: 1.3%, HR 0.443, 95% CI 0.119 to 1.650, p = 0.225).
Patients with STEMI receiving high-dose statin therapy had lower mortality than those receiving low dose or no statin therapy (high dose: 0.9% vs low dose or no statin: 4.1%, HR 0.221, 95% CI 0.077 to 0.636, p = 0.005; adjusted HR for high-dose statin 0.245, 95% CI 0.085 to 0.706, p = 0.009). This could not be demonstrated for patients with non-STEMI at presentation (high dose: 0.8% vs low dose or no statin: 3%, HR 0.256, 95% CI 0.070 to 0.929, p = 0.038; adjusted HR for high-dose statin 0.308, 95% CI 0.084 to 1.120, p = 0.075).
Patients aged <75 years receiving high-dose statin therapy had lower mortality than those receiving low dose or no statin therapy (high dose: 0.8% vs low dose or no statin: 2.9%, HR 0.269, 95% CI 0.101 to 0.717, p = 0.009; adjusted HR for high-dose statin 0.301, 95% CI 0.113 to 0.802, p = 0.016). This could not be demonstrated in the elderly (>75 years): high-dose statin 1.4% versus low dose or no statin 5.4% (HR 0.246, 95% CI 0.053 to 1.137, p = 0.072). These data are also graphically expressed in Figure 4 .




